340B provider and drug industry stakeholders joined yesterday in congratulating U.S. Public Health Service Lt. Cmdr. Emeka Egwim on his selection as Director of the U.S. Office of Pharmacy Affairs, the federal agency that runs the 340B drug pricing program.
…Category: Federal
A Democratic push in Congress to cap insulin costs for patients is gaining momentum. It may be a sign that some in the party are giving up on trying to pass comprehensive drug pricing legislation and focusing now on trying
…Breaking News
HRSA Picks Federal HIV/AIDS Policy Specialist Emeka Egwim To Be its Next 340B Program Director
Emeka Egwim, a federal government HIV/AIDS policy specialist who also has served in the Medicaid Drug Rebate Program, is the new head of the federal 340B Drug Pricing Program.
In a letter this morning to its staff, the U.S. Health
…The U.S. Health Resources and Services Administration’s (HRSA) recent statement that only three hospitals so far have said on a federal form that they lost their 340B eligibility due to fallout from COVID-19 does not reflect the problem’s much bigger
…The National Rural Health Association (NRHA) asked U.S. Health and Human Services (HHS) Secretary Xavier Becerra this week to act against drug manufacturers that deny 340B pricing when covered entities use contract pharmacies.
NRHA CEO Alan Morgan also urged Becerra
…A federal district judge in Florida has denied a group of defendants’ motion to dismiss Gilead’s lawsuit against them alleging fraudulent 340B-related transactions on the company’s HIV pre-exposure prophylaxis drugs Truvada and Descovy.
U.S. District Judge Aileen Cannon of the
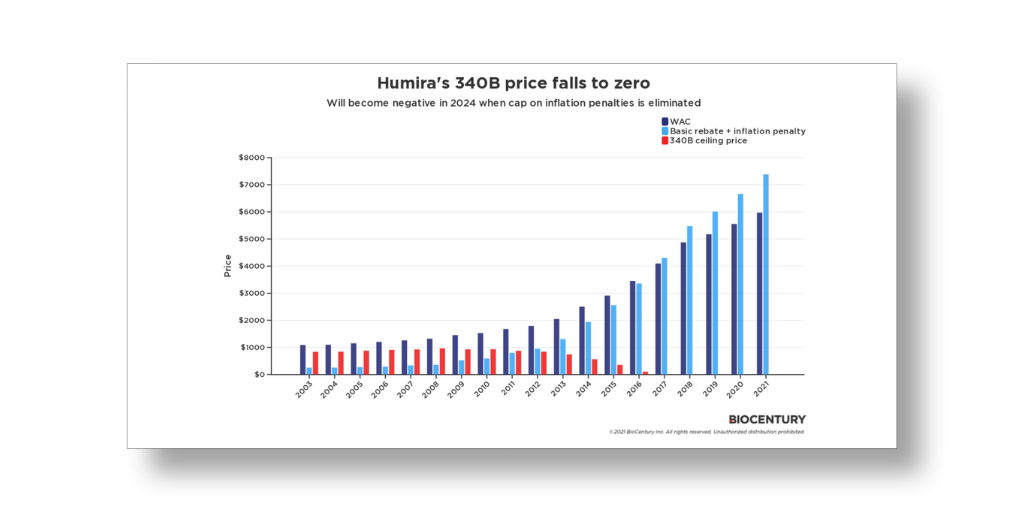
…In less than two years, brand drug companies that now must sell their products to 340B covered entities for a penny due to a history of price increases above inflation may have to start paying entities for their drugs instead
…UPDATED Thursday, Feb. 17, 2022, 4:00 p.m. EDT—The U.S. Health Resources and Services Administration (HRSA) said this afternoon, “The 340B program is an essential component of the safety net system that helps make health care and prescription drugs more affordable
…Insulin and diabetes drug manufacturers charged with federal and state price-fixing violations over their 340B contract pharmacy restrictions have again told a federal judge why they think she should dismiss the case.
AstraZeneca, Lilly, Novo Nordisk, and Sanofi filed their
…Drug manufacturer Merck is providing 340B covered entities with refunds for overcharges on 17 NDCs for purchases between Jan. 1 and March 31, 2019, the company says in a new public notice on the U.S. Health Resources and Services
…