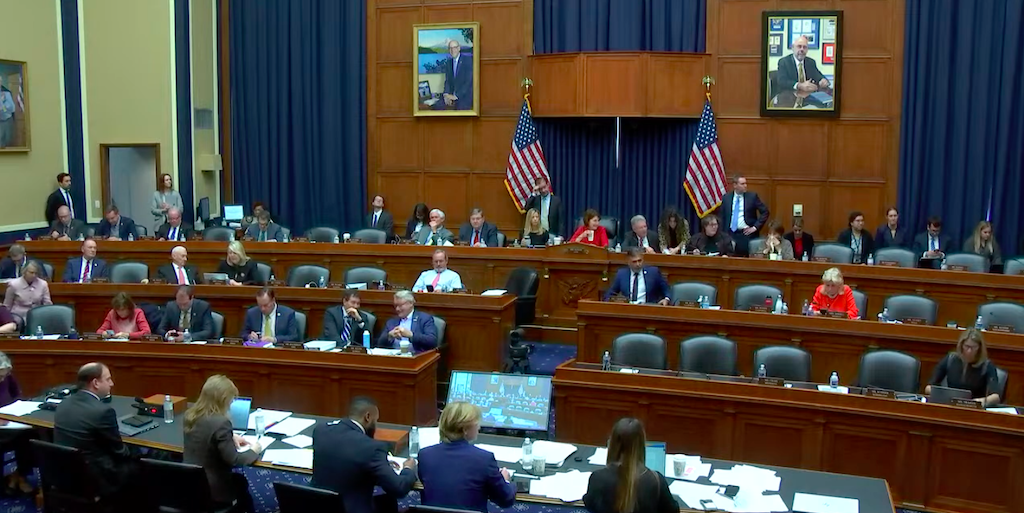
Lawmakers on a key House committee approved a pharmacy benefit manager (PBM) transparency bill on Wednesday that would require PBMs [...] …
Category: Federal
Two Republican Senators on an influential committee suggested during a hearing on Tuesday that 340B discounts could contribute to certain [...] …
A Senate Finance Committee Dec. 5 hearing on prescription drug shortages will include testimony from two witnesses who have written [...] …
The top Republican on an influential Senate committee today asked two community health centers to submit detailed financial information on [...] …
Hospital groups will meet with federal executive branch officials to discuss coming changes to the 340B program’s administrative dispute resolution [...] …
A group of Senate Republicans recently urged federal regulators to withdraw a plan to change the definition of a drug’s [...] …
News Alert
Novo Nordisk Files Suit to Block $100,000 Arkansas Fine for 340B Contract Pharmacy Violations
Danish drug giant Novo Nordisk filed suit Thursday to stop a proposed $100,000 fine from Arkansas over alleged violations of [...] …
The two hospital systems subject to a senator’s investigation into 340B program revenue have said they are fully compliant with [...] …
The federal government narrowly avoided a shutdown this week after lawmakers scrambled to pass a stopgap bill hours before government [...] …
The top Republican on an influential Senate committee has opened an investigation into how two major health systems use revenue [...] …