The National Association of Community Health Centers says in a new advocacy document “it’s time to reform the 340B program to recognize the complexities of today’s healthcare system and ensure that true safety-net providers continue to have access to critical
…Month: March 2023
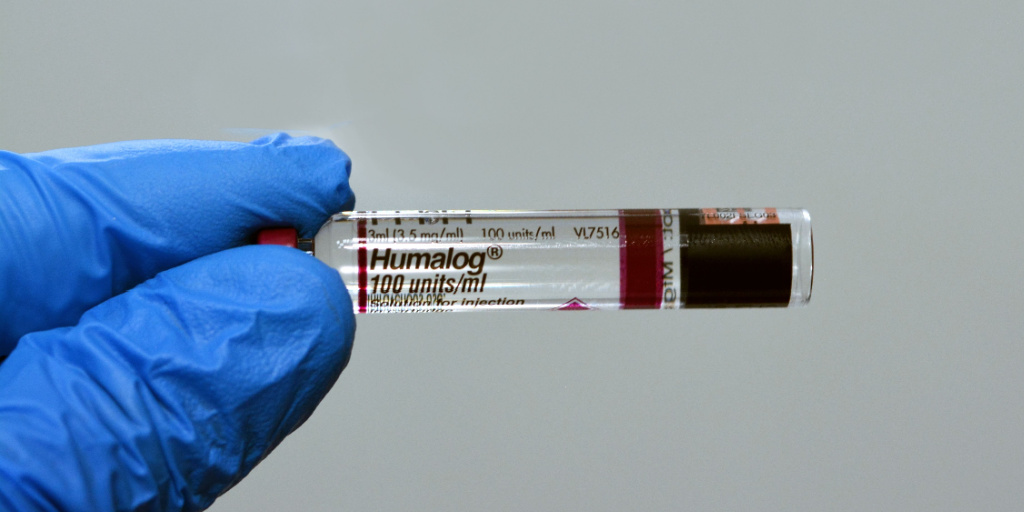
Drug manufacturer Lilly’s announcement last week that it is slashing the price of Humalog, its most commonly prescribed insulin, may have been less about altruism and more about avoiding exposure to bigger Medicaid rebates, a prominent drug pricing expert says.
…Generic drug manufacturer Zydus Pharmaceuticals USA said in a recent public notice it will pay refunds for 340B overcharges that occurred mostly in the first two quarters of 2022. The products include Zydus’ equivalents of the widely prescribed antibiotic Zithromax,
…The 340B program—and whether it needs change, protection, or both—came up often today during a U.S. Senate hearing on federal funding of community health centers.
The bulk of health centers’ federal grant funding—about $5.8 billion annually from the U.S. Health
…Associations representing community health centers and brand drug manufacturers are jointly developing 340B policy proposals for Congress to consider, hospital group 340B Health told its members yesterday.
The National Association of Community Health Centers said yesterday that it had no
…UPDATED Thursday March 2, 2023, 12:30 p.m. EDT to include comment from HRSA.
The U.S. Health Resources and Services Administration has asked multiple hospitals to explain how they determine patient eligibility for 340B drugs, how they define, track, and use
…SPONSORED CONTENT
Will you be attending the 340B Winter Coalition Conference in San Diego? Start your week with the latest developments in the 340B program. This year, Verity Solutions will be hosting a panel discussion on Monday morning with some of the most respected long-term experts on the 340B program, including Ted Slafsky, Publisher & CEO of 340B Report and Bill von Oehsen, Principal of Powers Law. Join us for an up-to-the-minute report on hot button topics related to the legislative, legal and regulatory aspects of the 340B program. Among the matters to be covered:
- The latest developments in the contract pharmacy battle and what to expect next in the courts
- An update on 340B legislative efforts in Washington, D.C. and the states including:
- Efforts to address contract pharmacy impasse
- Hospital transparency and good stewardship
- Potential for Congressional hearings
- Update on
The federal government may ask all 14 judges on the U.S. Third Circuit Court of Appeals to review a three-judge panel’s unanimous January opinion upholding AstraZeneca, Novo Nordisk, and Sanofi’s restrictions on 340B pricing when their drugs are shipped to
…