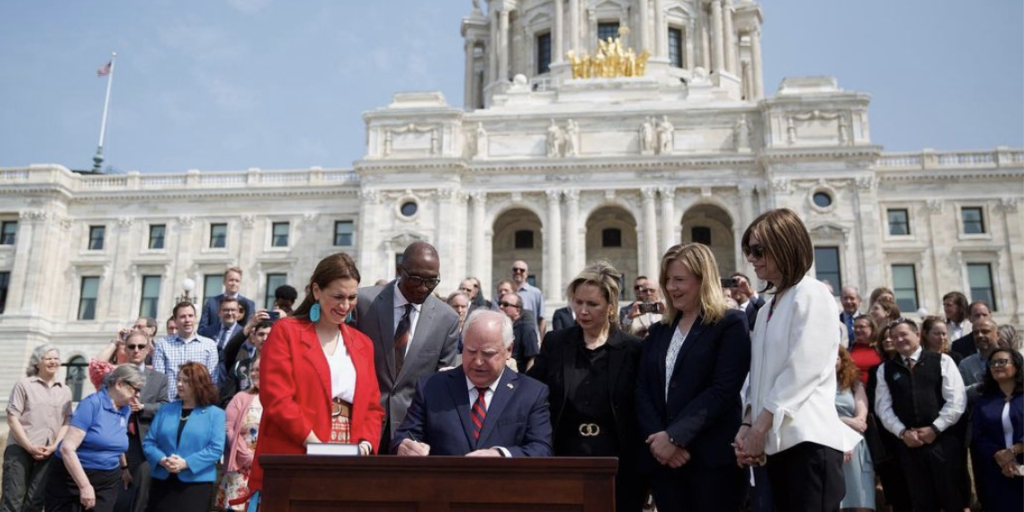Drug manufacturer Boehringer Ingelheim announced this morning that, starting Aug. 1, all covered entity types will be subject to its conditions on 340B pricing when entities use contract pharmacies to dispense BI products. Until now, its conditions have applied to
…Month: June 2023
Federal health care officials can’t make a South Carolina health center obey their reading of the 340B patient definition for the same reason a federal appeals court said in January that the officials can’t force drug manufacturers to deliver 340B
…Minnesota health care providers were shocked to learn that a bill signed into law in late May made their state the first to require all 340B covered entities to report their total 340B drug acquisition costs and payments.
The first
…Louisiana Gov. John Bel Edwards (D) has signed legislation making his state just the second with a law addressing manufacturer conditions on deliveries of 340B-acquired drugs to contract pharmacies.
Arkansas’s Act 1103, passed in August 2021, was the first. A
…Drug industry trade group Pharmaceutical Research and Manufacturers of America and drug manufacturer Bristol Myers Squibb have filed separate federal lawsuits to stop Medicare from negotiating the prices of some drugs, charging that it would impose unconstitutional price controls on
…
SPONSORED CONTENT

TIP: To ensure you’re not leaving any 340B dollars behind, assess your ineligible claims to understand if claims are being missed.
The six U.S. senators who asked Friday morning for ideas on ways to improve the 340B program elaborated on their reasons why in a news release that afternoon.
Sens. John Thune (R-S.D.), Debbie Stabenow (D-Mich.), Shelley Moore Capito (R-W.Va.), Tammy
…Ryan White Clinics for 340B Access yesterday criticized drug manufacturer Gilead Sciences for sponsoring a study that said it is unclear if patients of Ryan White clinics and other 340B grantee covered entities benefit from the drug margin these entities
…The American Hospital Association has asked the U.S. Health Resources and Services Administration to resume letting hospitals dispense 340B drugs at offsite clinics that have not yet appeared on the hospital’s most recently filed Medicare cost report.
AHA asked HRSA
…The Medicare Payment Advisory Commission is sticking by its long-standing recommendation that Medicare should pay less for Part B drugs furnished by 340B hospitals.
“We continue to believe that this approach is appropriate, and the specific level of payment reduction
…*Sign up for news summaries and alerts from 340B Report