Drug manufacturer Merck recently loosened its 340B program contract pharmacy restrictions in Louisiana and Arkansas just before Louisiana’s law protecting [...] …
Month: August 2023
Senate Appropriators Urge HRSA to Penalize Drug Makers That Impose 340B Contract Pharmacy Conditions
Federal health care officials should use fines and “any available measures” to punish drug makers that deny 340B pricing for [...] …
SPONSORED CONTENT
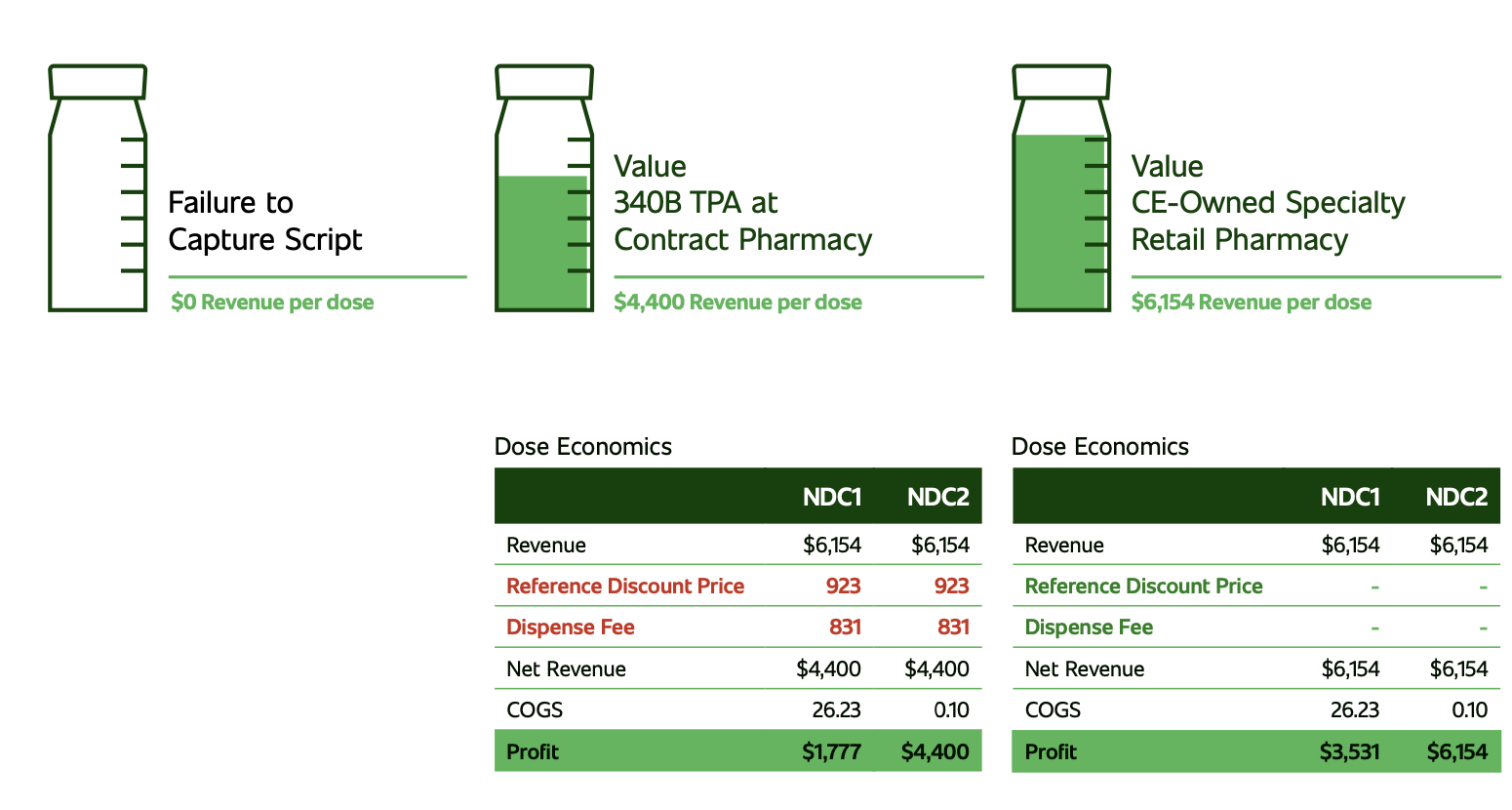
Today’s U.S. health systems face significant financial challenges. Many are still trying to recoup pandemic-related losses stemming from forced shutdowns, elective procedure postponements, and reduced outpatient visits. These factors have contributed to billions in lost revenue, leaving many of
…
Twenty-three state attorneys general urged a group of U.S. senators crafting a legislative overhaul of the 340B program to add [...] …
A high-profile researcher and colleagues are urging a range of changes to the 340B program, including new provisions on transparency, [...] …

Q: Where did you grow up?
I grew up in San Antonio and Victoria, TX and currently live in the Denver, CO area.
Q: Where did you go
…
Today is the first day of Louisiana’s new law protecting 340B program contract pharmacy arrangements and there have already been [...] …
The creation of a claims clearinghouse and increasing both the funding and authority of the federal agency overseeing the 340B [...] …
Republican leaders of the U.S. House Energy and Commerce (E&C) Committee released a draft bill late last week that would [...] …
The U.S. Health Resources and Services Administration (HRSA) still stands by its 340B program patient definition, the agency said in [...] …