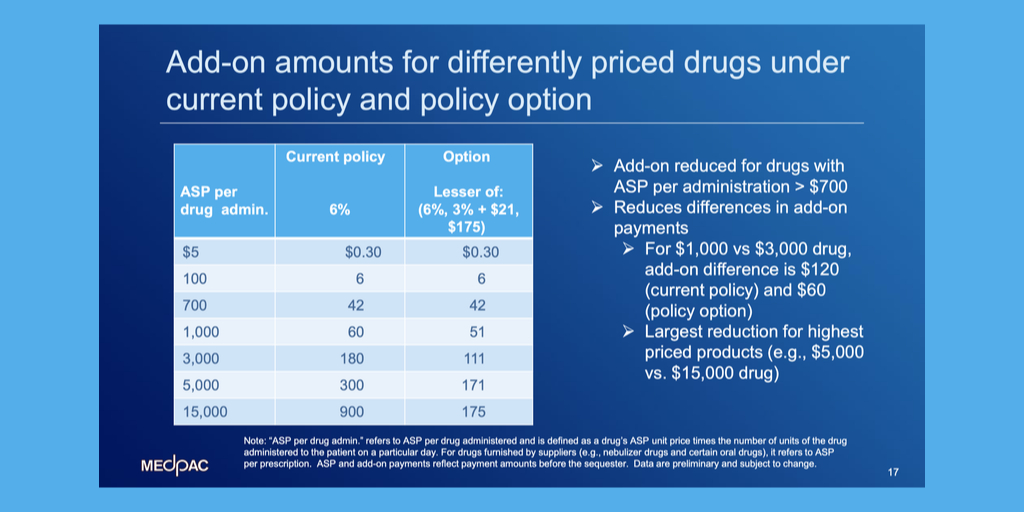
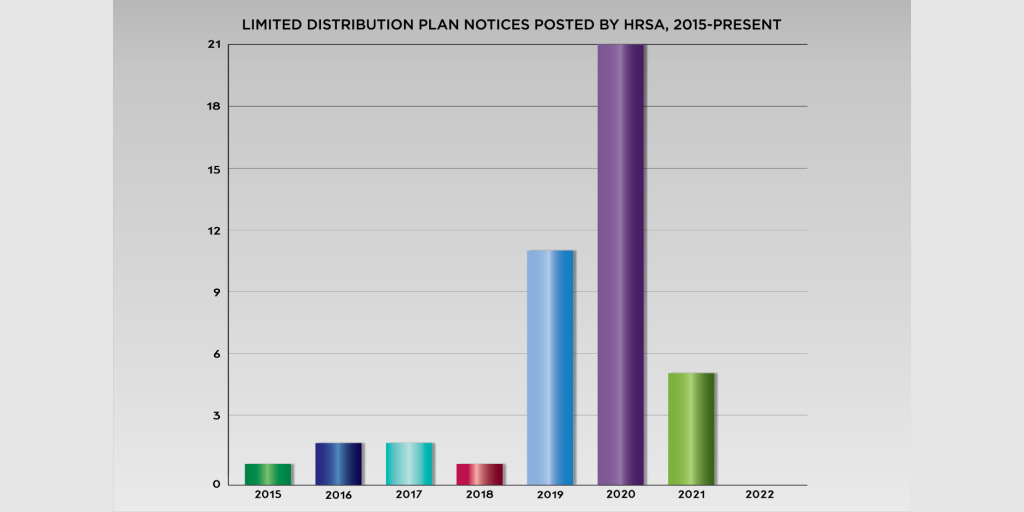
A federal district judge in Washington, D.C., yesterday ordered community health centers to answer why she should not dismiss their lawsuit against federal health officials over the administrative dispute resolution process for the 340B program.
Judge Florence Pan of U.S.
…