In a decision that could have significant implications for 340B stakeholders, a federal district judge in Indianapolis today granted drug manufacturer Eli Lilly’s motion for a preliminary injunction stopping the U.S. Health and Human Services Department from implementing or enforcing
…Month: March 2021
Correction, Tuesday March 16, 4:00 p.m. EDT—Earlier today, we reported that during a March 16 webinar, advisers to 340B covered entities said that U.S. Rep. Abigail Spanberger (D-Va.) had written to pharmacy benefit manager Express Scripts (ESI) about its new
…Federally qualified health centers and their trade groups are adamantly opposed to implementing a Trump administration rule to require health centers to provide insulin and injectable epinephrine to low-income patients at the price the centers pay for those drugs under
…Medicare Part B drug payments to 340B hospitals “declined substantially” in 2019 because the payment status of “an unusually high number of drugs” changed that year, congressional Medicare advisers said in a report yesterday.
The change—from “pass-through status” to “separately
…President Biden has nominated Melanie Egorin, a veteran Democratic congressional health care adviser, to serve as U.S. Health and Human Services (HHS) Assistant Secretary for Legislation.
If confirmed, Egorin would be a top deputy to Xavier Becerra, whose nomination to
…Pharmacy benefits manager Express Scripts’ (ESI) new pharmacy reporting requirement for reclassifying a previously submitted prescription drug claim as 340B-eligible “applies to the extent not prohibited by law,” the PBM says in an FAQ on its website.
ESI’s statement in
…At least six states have introduced or passed bills in recent weeks that would forbid pharmacy benefit managers from discriminating against 340B covered entities regarding reimbursements and other practices.
The bills range from 340B-specific legislation to broader bills to address
…The Biden administration proposes once again to push back, this time from March 22 to July 20, the effective date of the Trump administration’s controversial final rule to require health centers to provide insulin and injectable epinephrine to low-income patients
…Drug manufacturer Sanofi on Monday struck back at the U.S. Justice Department (DOJ) for opposing its Feb. 2 motion in a federal lawsuit requesting an injunction against the 340B program’s new administrative dispute resolution (ADR) process.
“The government is eager
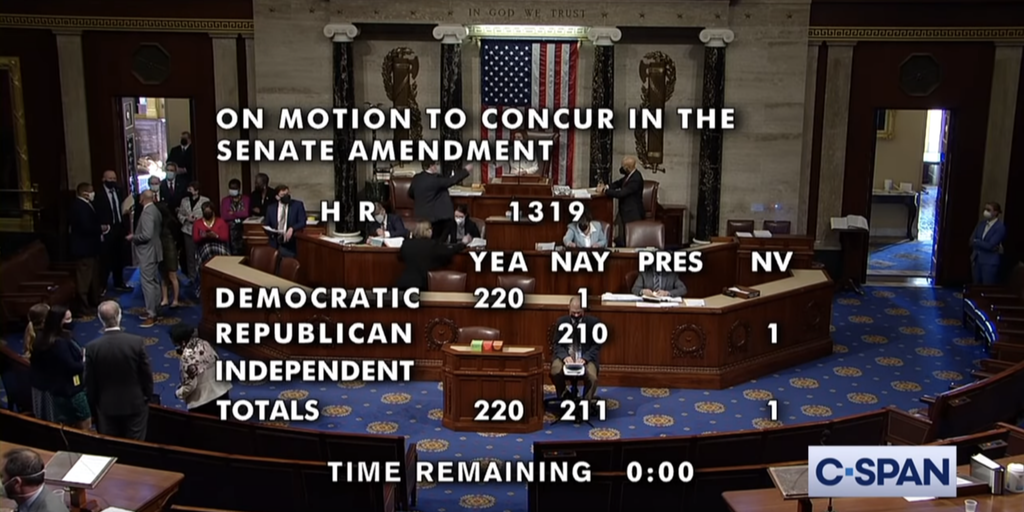
…The U.S. House on Wednesday passed and sent to President Biden a $1.9 trillion COVID-19 relief and economic recovery bill with provisions affecting the 340B program and its participants. Biden will sign the bill at the White House tomorrow.
The
…