The U.S. House Appropriations Committee said this week it is concerned about the growing number of drug manufacturers “that curtail the use of 340B drugs in contract pharmacies.” It encouraged the U.S. Health Resources and Services Administration to use “any
…Month: June 2022
Drug manufacturer UCB says it is reviewing Monday’s letter from the U.S. Health Resources and Services Administration telling the company its restrictions on 340B pricing to covered entities that use contract pharmacies break federal law and must end immediately.
“At
…The American Hospital Association wants to discuss with U.S. Health and Human Services Secretary Xavier Becerra how 340B hospitals should be promptly repaid “for all of the years (2018-2022) in which the Centers for Medicare & Medicaid Services (CMS) illegally
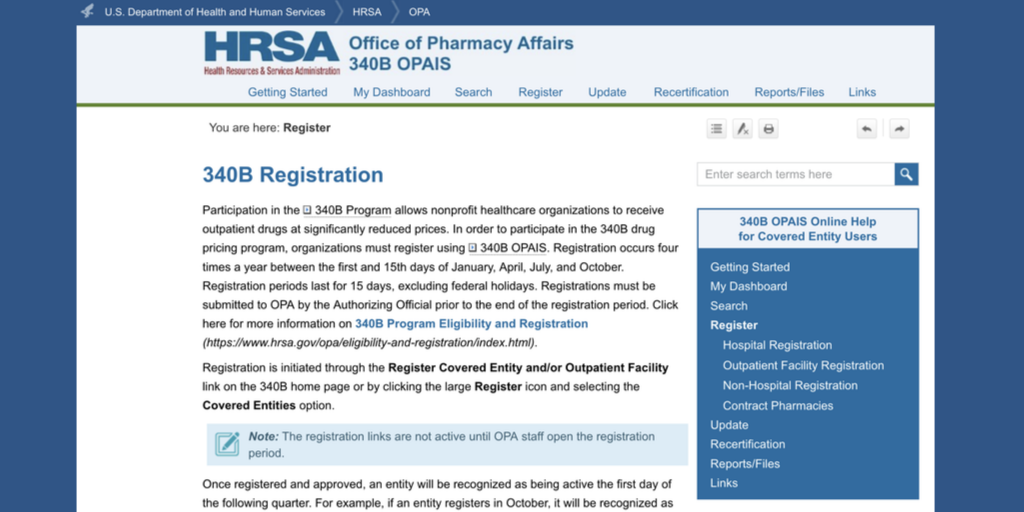
…The third quarterly period this year for 340B registration of new covered entities, outpatient facilities, and contract pharmacies will begin tomorrow, July 1, and end on Friday, July 15.
Eligible hospitals, health centers, clinics, and their child sites and contract
…
340B PROVIDER LEADER SPOTLIGHT
Jessica Rivas, Chief Pharmacy Officer for Big Bend Cares and Scholarship Recipient for the Apexus Advanced 340B Operations Certificate Program

Q: Where did you complete your professional training?
I completed my pharmacy technician program during my senior year in high school at Westside Tech in Orlando, Florida. I
…Federal healthcare officials told drug manufacturer UCB yesterday its restrictions on 340B pricing when covered entities use contract pharmacies are illegal and must stop or the company could face civil monetary penalties of up to $6,323.00 for each instance of
…A federal district judge was right to decide that drug manufacturer Lilly cannot tie strings to 340B pricing on its drugs, the U.S. Justice Department told a federal appeals court in Chicago on Friday. But, DOJ said, the judge was
…One of two groups of Florida health care providers that drug maker Gilead accused of making fraudulent 340B-related transactions involving the company’s HIV pre-exposure prophylaxis drugs agreed last week to settle Gilead’s claims against it for $33 million.
A mediation
…A U.S. Supreme Court decision last week about the Medicare disproportionate share adjustment percentage calculation will have little effect on hospitals currently in the 340B drug pricing program, experts say. It could mean, though, that some on the cusp of
…