The U.S. Supreme Court’s unanimous June 15 ruling that huge cuts in 340B hospitals’ Medicare Part B drug reimbursement were unlawful “is a clear and much welcome victory for 340B hospitals,” 340B Report Publisher and CEO Ted Slafsky says in
…Year: 2022
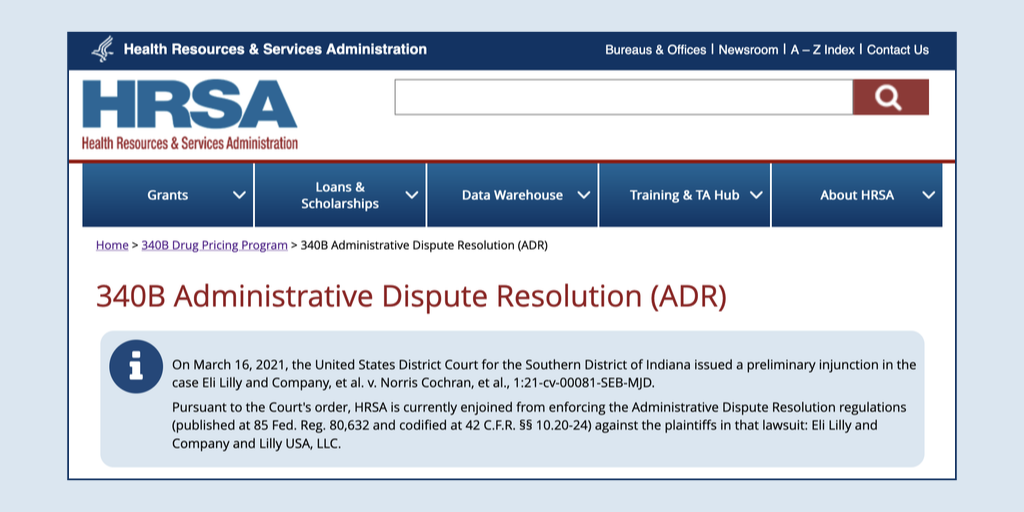
The White House office that oversees issuance of federal healthcare regulations now says that this is the target month for releasing a long delayed proposed rule to replace 340B program dispute resolution regulations. But the administration has not given a
…U.S. Sens. Rick Scott (R-Fla.) and Ben Ray Luján (D-N.M.) have introduced a bill to let Ryan White clinics use income from being in the HIV/AIDS program to provide pre-exposure prophylaxis, or PrEP, a daily medication used to prevent people
…Drug manufacturer Gilead on Wednesday added 45-day time limits for replenishment orders and claims submissions to its conditions on 340B pricing when covered entities use contract pharmacies.
Ten manufacturers require covered entities to provide claims data to industry contractor 340B
…SPONSORED CONTENT
Community health center (CHC) pharmacies, which have long operated with tight budgets, have recently been further weighed down by a trifecta of woes:
- Significant staffing shortages and lower margins related to COVID-19 – according to a 2021 Capital Link report, 66% of CHCs risked
UPDATE Wednesday, June 22, 2022, 2:50 p.m. EDT—CVS Health issued this statement: “We do not comment on specific PBM client contract terms. Our clients are sophisticated purchasers, and we work closely with them to customize a benefit design meeting their
…The federal government told a federal appeals court in Philadelphia yesterday it should use “the familiar tools of statutory interpretation” to hold that drug manufacturer AstraZeneca may not unilaterally dictate how many contract pharmacies a 340B covered entity may use
…West Virginia has reinforced its groundbreaking 2019 law forbidding what providers say is pharmacy benefit manager discrimination against 340B covered entities.
An amendment passed in March that took effect June 10 clarifies that PBMs or other third-party payers may not require a
…Growth in 340B covered entities’ use of contract pharmacies to dispense 340B drugs, coupled with growth in pharmacy benefit managers’ control over these pharmacies, has given PBMs an outsized, profit-driven role in the 340B program that does not benefit patients,
…News Alert
NACHC Asks HHS to Swiftly Punish Drug Makers for 340B Pricing Denials Involving Contract Pharmacies
The National Association of Community Health Centers (NACHC) strongly urged the U.S. Health and Human Services Secretary Xavier Becerra yesterday “to swiftly impose” fines on drug manufacturers that restrict shipments of 340B-purchased drugs to contract pharmacies and “hold manufacturers accountable
…