Letting drug manufacturers put conditions on 340B drug discounts “would be akin to letting the fox guard the henhouse,” the U.S. Justice Department (DOJ) told federal appeals courts in Philadelphia and Washington, D.C., last night in a pair of filings
…Year: 2022
Drug manufacturer Amgen is providing refunds for overcharges during varying periods over three years on some of its top selling drugs including Enbrel ($4.8 billion in U.S. sales in 2020), Neulasta ($2 billion), Kyprolis ($710 million), Aranesp ($629 million), Epogen
…America’s oldest consumer protection group, shunned by some former allies in the labor movement for alleged favoritism toward its corporate donor Amazon, has slammed the 340B program in a commentary for the conservative-leaning news and opinion website RealClearHealth.
“Sadly, as
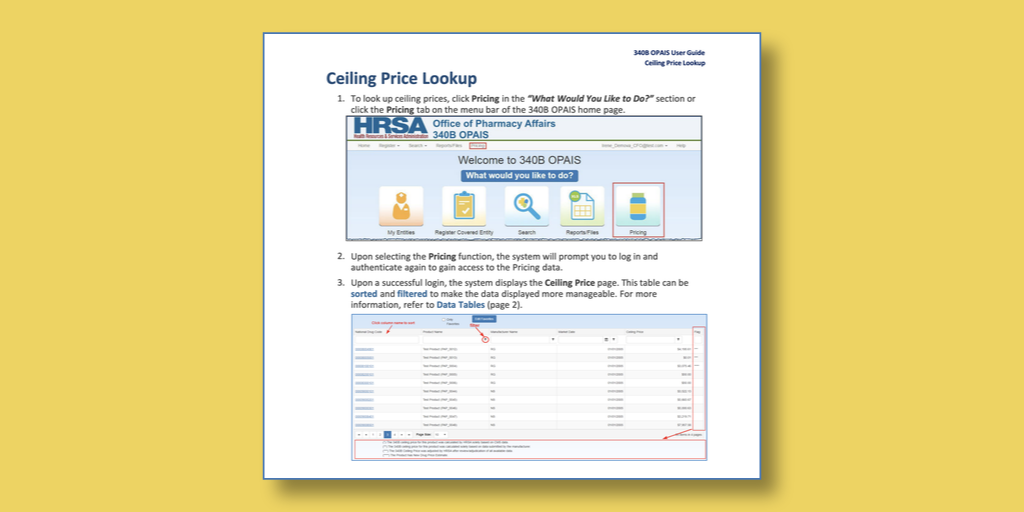
…After a nine-year delay, federal officials in April 2019 did as Congress ordered and began posting 340B ceiling prices on a secure website. For the first time, providers enrolled in the 340B program had a tool to help them make
…Breaking News
HRSA Sends Merck 340B Contract Pharmacy Cease and Desist Letter, Agency’s First in Seven Months
The federal government told drug manufacturer Merck today its conditions on 340B pricing when hospitals and health centers use contract pharmacies are illegal and must stop, or the company could face penalties of up to $6,323 per each instance of
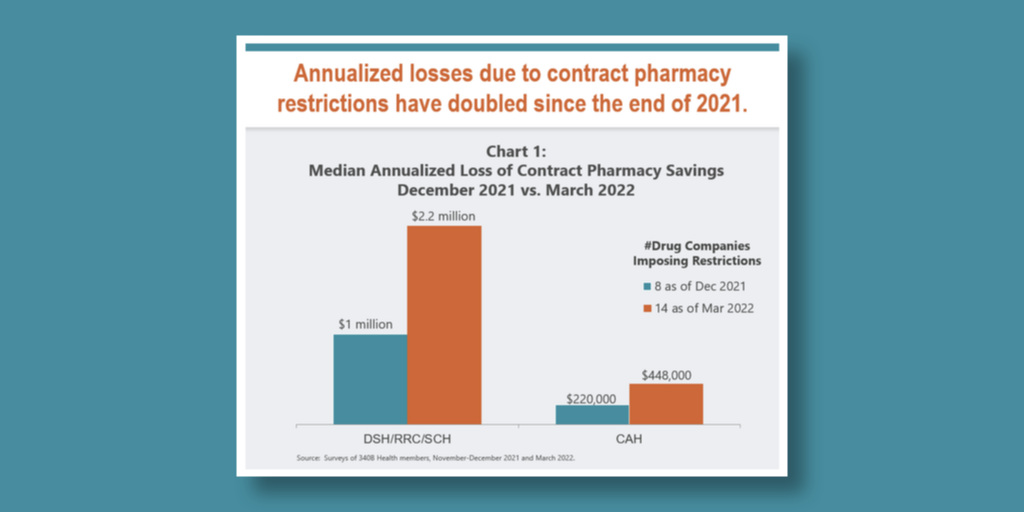
…The financial impact to 340B hospitals due to drug manufacturers’ restrictions on contract pharmacy more than doubled between December 2021 and March 2022, a new survey shows.
Hospital trade group 340B Health said the median annualized loss of contract pharmacy
…A federal district judge on Tuesday let Arkansas’ state health center association and a critical access hospital in the state have a role in the drug industry’s lawsuit challenging the state’s one-of-a-kind law protecting 340B contract pharmacy arrangements.
U.S. Senior
…Drug manufacturer AstraZeneca effective June 1 will stop offering voluntary 340B pricing on its orphan drugs to critical access hospitals, sole community hospitals, rural referral centers, and free-standing cancer hospitals, the company said yesterday.
“AstraZeneca is making this change to
…California health centers asked a federal district judge in late April to deny the U.S. Centers for Medicare & Medicaid Services (CMS) motion to dismiss the health centers’ claims against CMS arising over the state’s transfer of Medicaid managed care
…