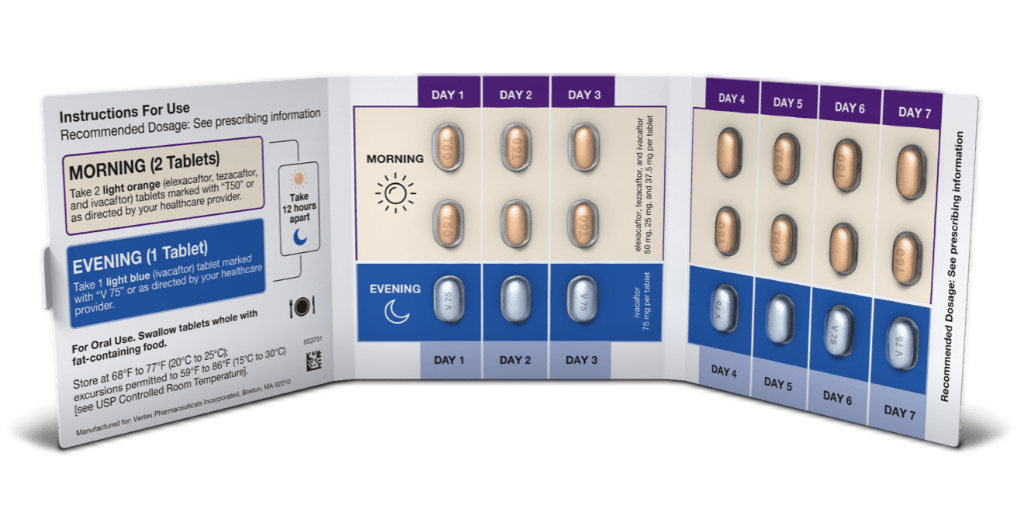
Biopharmaceutical company Vertex is providing refunds to 340B covered entities for overcharges on its cystic fibrosis (CF) combination medicine Trikafta for the period from Q2 2021 through Q4 2021, according to a public notice on the U.S. Health Resources and
…Year: 2022
340B Report Publisher and Chief Executive Officer Ted Slafsky says in his latest column for Omnicell’s blog that “all signs foretell a Republican takeover of the House and a very good possibility of the GOP assuming control of the closely
…UPDATE Friday, Jan. 28, 2022, 3:25 p.m.—The U.S. Health Resources and Services Administration (HRSA) said this afternoon it is reviewing Pfizer’s policy and will evaluate next steps as needed.
Pfizer today became the 13th drug manufacturer to impose conditions on
…The U.S. Health Resources and Services Administration (HRSA) told drug manufacturer Celgene nearly five years ago the company “must ensure” that covered entities could buy its cancer drugs Revlimid, Pomalyst, and Thalomid at the 340B ceiling price through Celgene’s limited
…Drug manufacturer Gilead will voluntarily extend 340B pricing to critical access hospitals, sole community hospitals, and rural referral centers on Veklury (remdesivir), its injectable antiviral treatment for COVID-19, the company said late Tuesday.
The U.S. Food and Drug Administration (FDA)
…UPDATE Friday, Jan. 28, 2022—This story was updated to include comments by Pharmaceutical Research and Manufacturers of America (PhRMA.)
340B hospitals “are losing significant resources” due to drug companies’ conditions on 340B pricing when covered entities use contract pharmacies, according
…Health care services and group purchasing company Vizient asked Congress on Monday to pass the U.S. Senate version of bipartisan legislation to protect hospitals from losing their 340B eligibility due to changes in patient and payer mix caused by the
…
340B PROVIDER LEADER SPOTLIGHT
Julie Valdes, Clinical Pharmacist & 340B Program Coordinator, Zufall Health Centers Inc.

Q: Where did you go to college/graduate school?
For college, I went to Occidental College in Los Angeles, California and majored in Biochemistry. For graduate school, I went
…Three Democratic and three Republican U.S. House members asked the U.S. Health and Human Services Department (HHS) on Friday “to move quickly to enforce the law and begin assessing civil monetary penalties on manufacturers that have denied 340B pricing to
…