The U.S. Centers for Medicare & Medicaid Services said late this afternoon that it will address how it will remedy five years of illegal Medicare Part B underpayments to hospitals for 340B purchased drugs “in future rulemaking,” rather than in
…Year: 2022
The U.S. Health Resources and Services Administration, for now at least, is setting aside a proposed change to 340B program forms that a provider group warned might lead to covered entities losing access to 340B pricing under some drug manufacturers’
…A South Carolina health center and the federal government are sparring over whether the center should have access to more information from the government than it already possesses in its fight to clarify the 340B patient definition.
Oct. 26 court
…Getting 340B pharmacy data to study trends and public health impacts “is like trying to catch a will-o’-the-wisp,” a former senior Trump administration health care official said in a commentary last week in the life sciences news publication STAT.
“The
…Breaking News
Appeals Court Questions Yet Again if it Has Jurisdiction Over Lilly’s 340B Contract Pharmacy Case
A federal appeals court judge in Chicago expressed doubt during a hearing this morning that the court can review a lower court decision in drug manufacturer Eli Lilly’s 340B contract pharmacy lawsuit.
Judge Frank Easterbrook, the senior judge on the
…Now that a federal appeals court in Washington, D.C., has heard arguments in Novartis and United Therapeutics’ 340B contract pharmacy lawsuits, the scene shifts to Chicago, where an appeals court will hear arguments Monday morning in Eli Lilly’s companion case.
…AIDS Healthcare Foundation (AHF) members are staging a weeklong, twice-daily series of protests at drug maker Gilead Science’s Northern California headquarters to protest the company’s policy to impose conditions on 340B providers that use contract pharmacies to dispense 340B-priced drugs.
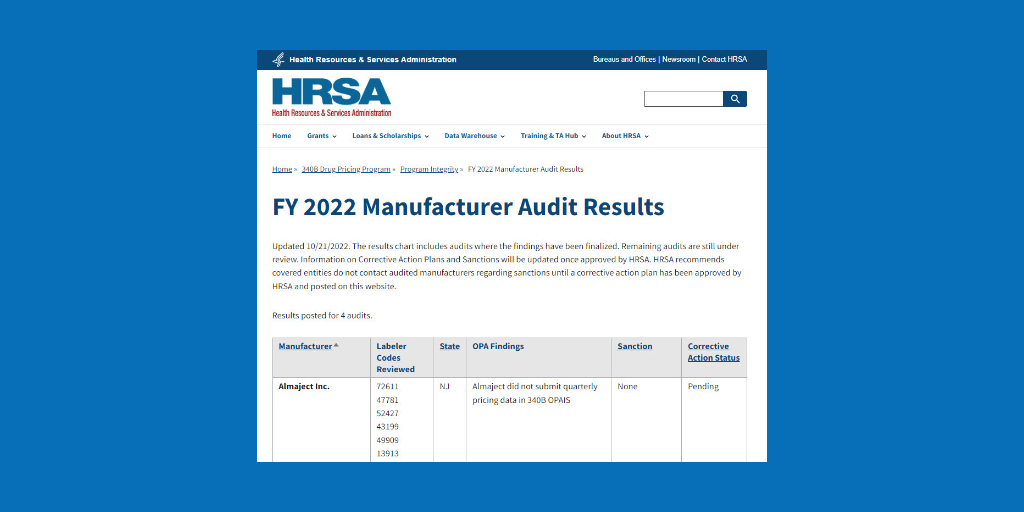
…The U.S. Health Resources and Services Administration (HRSA) has cited Almaject, a New Jersey-based maker of generic injectables, with failing to submit quarterly pricing data into the 340B Office of Pharmacy Affairs Information System, although the agency did not impose
…Two of three judges on a federal appeals court panel yesterday repeatedly asked lawyers during a highly anticipated hearing in Washington, D.C., what standard the court should use to decide whether drug manufacturers’ 340B contract pharmacy conditions are legal.
The
…Congress’ nonpartisan research office has issued a four-page backgrounder on litigation over the 340B contract pharmacy program and a two-pager on the entire drug discount program. They could be a sign that 340B hearings and/or bills are coming.
The Congressional
…