The U.S. Health Resources and Services Administration midday yesterday and the 340B prime vendor Apexus this morning, in connection with the end of the COVID-19 public health emergency late last night, withdrew mostly identical 340B program FAQs about use of
…Year: 2023
U.S. Sen. Mike Braun (R-Ind.) did not seek to amend a PBM oversight bill that is being marked up today to require 340B hospitals and possibly other covered entities to be more transparent about how they use money saved or
…A Louisiana Senate health committee yesterday passed a bill that will stop drug manufacturer interference with 340B contract pharmacy arrangements, rejecting an amendment that would have stripped the language from the measure.
HB 548, which also would prohibit pharmacy benefit
…Drug manufacturer Janssen Pharmaceuticals said last week it would pay refunds for overcharges on 340B drugs purchased in the second quarter of 2020, including top-selling psoriasis drug Stelara.
Janssen, part of Johnson & Johnson, said it owes or may owe
…
Q: Where did you grow up?
Ho Chi Minh City, Vietnam
Q: Where did you go to college/graduate school?
Northeastern University for Pharmacy and Columbia University for Public Health
Q: What are your favorites?
Food: Anything that has a filling wrapped in dough – dumpling, empanada, ravioli!
Movie: I have seen Forrest Gump more
…
SPONSORED CONTENT

TIP: The key to unlocking the full potential of your program starts with complete understanding of your data.
Editor’s note: New information from HRSA and Apexus obtained as we were going to press appears at the end of this article.
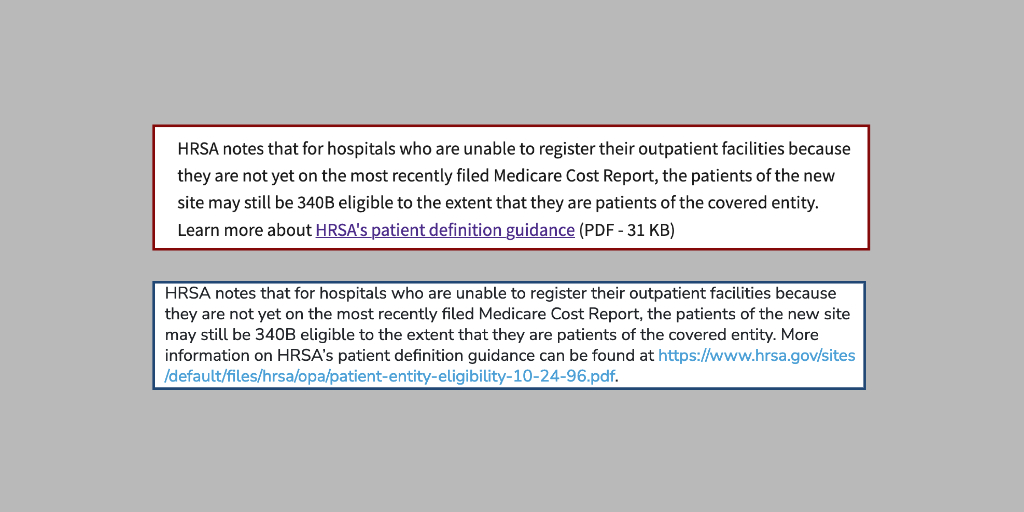
Hospital representatives said they were floored to learn yesterday that a June 2020 federal 340B patient eligibility policy
…A U.S. House Energy & Commerce subcommittee plans next week to markup bills on transparency and competition in health care and send them to the full committee for votes before Memorial Day. Although its Republican vice chair’s draft 340B bill
…Generic drug manufacturers Lifestar Pharma and Chartwell Pharmaceuticals may owe refunds to 340B covered entities for overcharges on drugs purchased from the companies in the fourth quarter of 2022, according to recent notices posted on the U.S. Health Resources and
…
Q: Where did you grow up?
Clifton Park, New York, a small suburb upstate, between Albany and Saratoga Springs, NY.
Q: Where did you go to college?
I entered the 6-year Doctor of Pharmacy program at Massachusetts College of Pharmacy and Health Sciences directly after high school. After completing my Pharm.D., I completed a PGY1 pharmacy
…