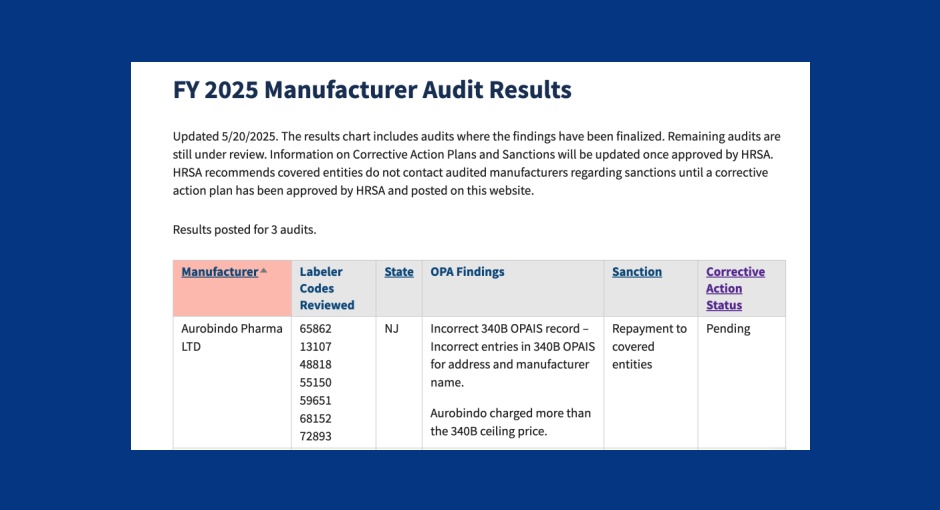
For the third straight time in 2025, a federal audit has found a drug manufacturer overcharged covered entities for 340B [...] …
Articles by : Bella Czajkowski, National Correspondent
A recent drug-industry-funded study attributes the 340B program’s recent growth to increased utilization—not rising drug prices. The May 21 study, [...] …
There’s a gap in economics research on the 340B program, and economists could enrich 340B stakeholders’ understanding of the program [...] …
Pharmaceutical giant Amgen recently announced it will exempt Tennessee providers from its contract pharmacy restrictions despite a carve out in [...] …
SpecGx, a generic subsidiary of Mallinckrodt Pharmaceuticals, will refund 340B covered entities that purchased addiction treatment medication Methadose for above [...] …
Editor’s Note: With 340B-related litigation heating up in several states following the enactment of new contract pharmacy access laws, 340B [...] …
Three drugmakers will refund covered entities that purchased certain 340B-priced medications following recent adjustments to their 340B drug ceiling prices. [...] …
An Indiana congresswoman recently introduced a controversial U.S. House bill that could potentially require 340B covered entities to pass all [...] …
Editor’s Note: With 340B-related litigation heating up in several states following the enactment of new contract pharmacy access laws, 340B [...] …
Three drugmakers recently announced refunds for 340B covered entities that were overcharged when buying certain drugs. Viatris, Amgen and GSK [...] …