The drug industry and 340B program advocates offered mixed reactions Monday to a key senator’s latest inquiries into drugmakers’ contract [...] …
Articles by : Bella Czajkowski, National Correspondent
Editor’s Note: This article is available to all and is not behind the paywall. The first episode of 340B Report’s [...] …
The U.S. House passed a bipartisan bill Tuesday evening to streamline Medicaid enrollment for pediatric providers that would also increase [...] …
Two drugmakers recently announced that they will refund 340B covered entities that purchased certain medications following adjustments to their ceiling [...] …
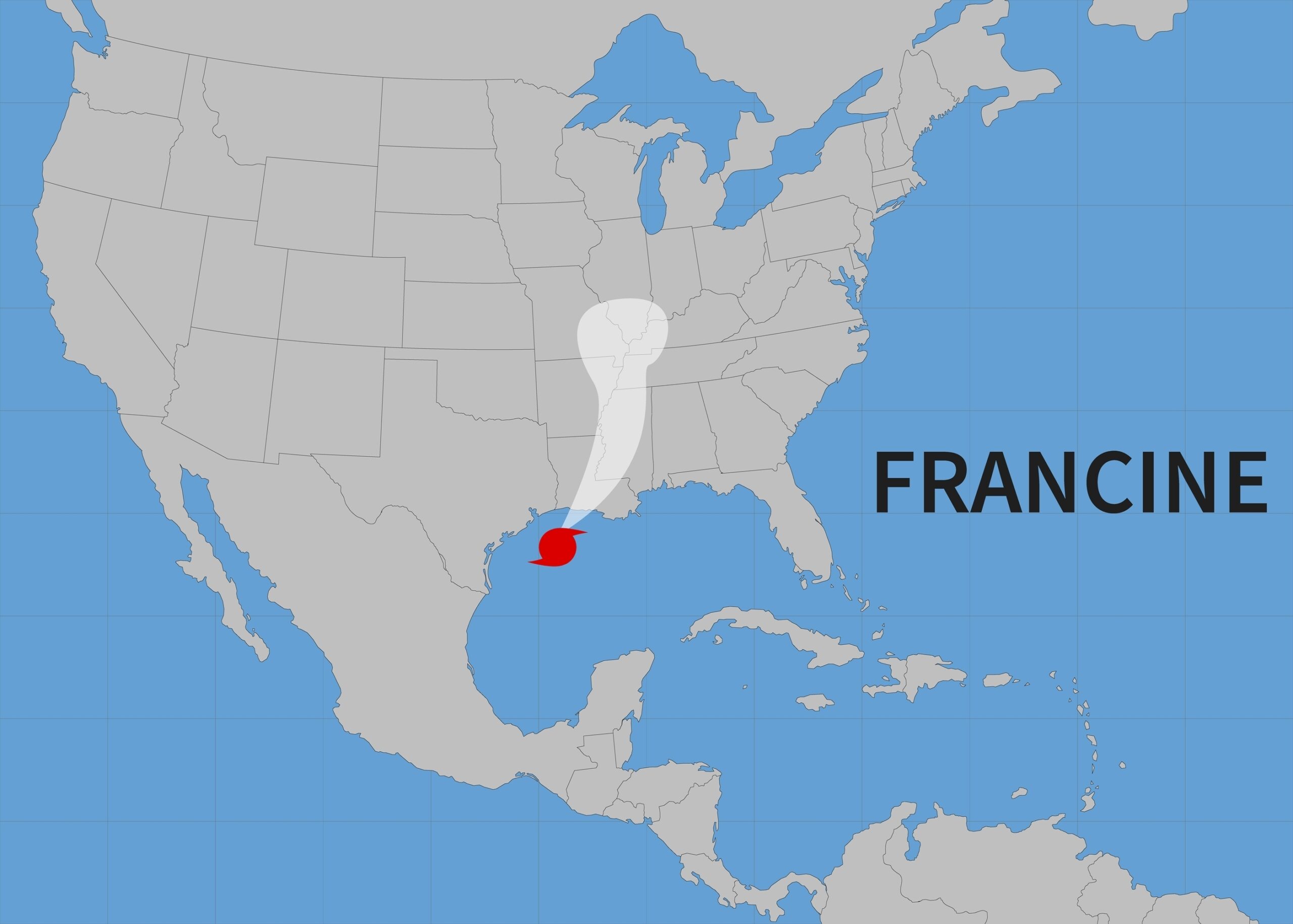
Louisiana providers can enroll in the 340B program outside of the formal enrollment period amid disaster relief efforts following Hurricane [...] …
Beginning Oct. 1, AstraZeneca will tighten its 340B contract pharmacy restrictions to require claims data on 340B utilization at contract [...] …
The 340B program has outgrown patient demands and requires additional restrictions and oversight, according to a new report from a [...] …
Specialty and community pharmacies play an essential role in the 340B program and must be protected against interference by drug [...] …
November will bring an epic battle for both the U.S. presidency and control in Congress, both of which could have [...] …
Johnson & Johnson (J&J) has ended its contract pharmacy restrictions for covered entities in Arkansas, making the New Jersey-based pharmaceutical [...] …