Multiple hospital advocacy groups have filed an amicus brief urging a federal district judge to uphold Nebraska’s recently enacted 340B [...] …
Articles by : Bella Czajkowski, National Correspondent
Three drugmakers recently announced they will exempt additional states from their 340B contract pharmacy restrictions in light of new contract [...] …
The 340B program is driving up employer-sponsored health insurance premiums, according to a drug industry-funded white paper. Provider advocates criticized [...] …
Four drugmakers recently announced they will exempt additional states from their 340B contract pharmacy restrictions in response to a flurry [...] …
Editor’s Note: With 340B-related litigation heating up in several states following the enactment of new contract pharmacy access laws, 340B [...] …
Federal appeals court judges on Friday questioned whether four major insulin manufacturers could have imposed similar 340B contract pharmacy restrictions [...] …
The U.S. Supreme Court on Tuesday sided against more than 200 hospitals in a dispute over Medicare disproportionate share hospital [...] …
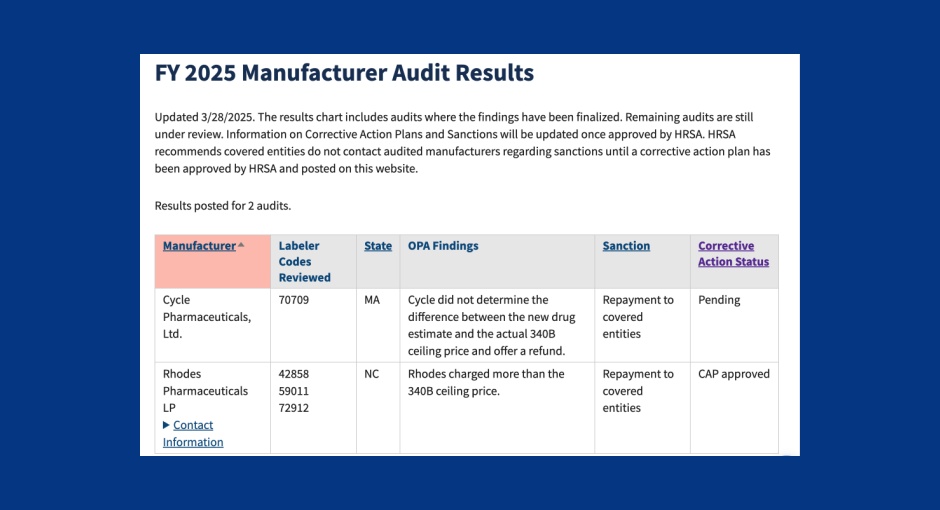
Recent Health Resources and Services Administration (HRSA) audits found that two drugmakers overcharged covered entities for their 340B drugs, fueling [...] …
The drug industry is bracing for the Trump administration’s planned tariffs on pharmaceutical products and ingredients, warning of supply chain [...] …
Minnesota covered entities will have additional 340B reporting requirements this year, but an extended deadline to submit that data, according [...] …