Drugmaker Novo Nordisk announced it is providing 340B covered entities 12-months-worth of refunds on four drug products including two of [...] …
Articles by : Rich Daly, Editor in Chief
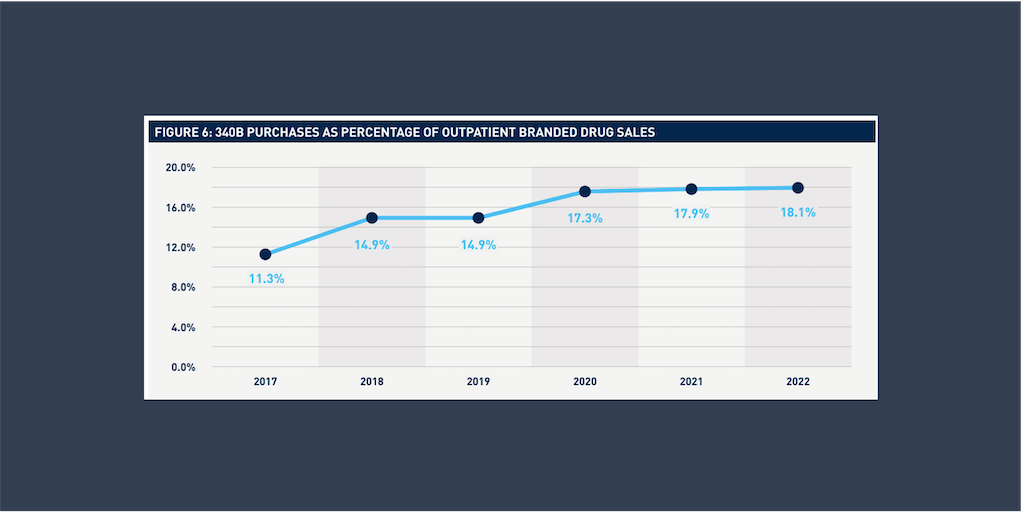
340B drugs have steadily increased as a share of U.S. branded outpatient pharmaceutical sales and reached 18% of the total [...] …
Hospitals in the 340B program should respond to increasing drugmaker contract pharmacy restrictions by opening their own pharmacies or selectively [...] …
CVS Health recently cited drug manufacturers’ contract pharmacy restrictions as one of the causes for a 19% year-over-year income decline [...] …
The first fiscal year 2024 340B provider audit reports included no adverse findings for 19 organizations. The May 8 audit [...] …
A California health system’s lawsuit alleging “hundreds of millions” in overcharges by four drugmakers was recently released from required mediation [...] …
Recently released draft guidance for implementing Medicare price caps would allow voluntary provider use of 340B codes on claims, while [...] …
Drug spending increased by 13.6% in 2023 to $722.5 billion, according to recent research. It found divergent trends for clinic [...] …
Federal auditors closed audits with corrective action plans (CAPs) for eight more 340B providers, which brought the fiscal year 2023 [...] …
Editors’ note: After publication, Pfizer became the 24th drugmaker to loosen or end its contract pharmacy restrictions for Arkansas covered entities, [...] …