After an initial burst of optimism earlier this year, some health care organizations are having second thoughts about large in-person events for the remainder of 2021 and the start of the new year. However, some groups including the 340B Coalition
…Articles by : Ron Shinkman,
Hospital trade groups are lodging strong objections to maintaining steep cuts in Medicare Part B drug reimbursement as part of the Fiscal Year 2022 final rule for the hospital outpatient prospective payment system (OPPS), while the trade group for brand
…The U.S. Health Resources & Services Administration (HRSA) today referred six drug manufacturers to the U.S. Health and Human Services Department of the Office of the Inspector General (HHS OIG) regarding their refusal to offer 340B discounts to 340B covered
…The dispute with pharmaceutical firms over the use of contract pharmacies for 340B covered entities is the biggest crisis facing the program since its inception in the early 1990s, 340B Report Publisher and CEO Ted Slafsky tells “The Future of
…North Carolina Gov. Roy Cooper (D) signed into law yesterday a bill that imposes greater oversight of pharmacy benefit managers and protects most categories of 340B entities from so called discriminatory reimbursement and contracting practices. Disproportionate share hospitals (DSH), children’s
…With the deadline for hospitals to recertify their eligibility for 340B drug discounts arriving on Monday, it appears most facilities will remain qualified—at least for now.
Industry observers say the COVID-19 pandemic’s impact on hospitals’ eligibility for 340B could become
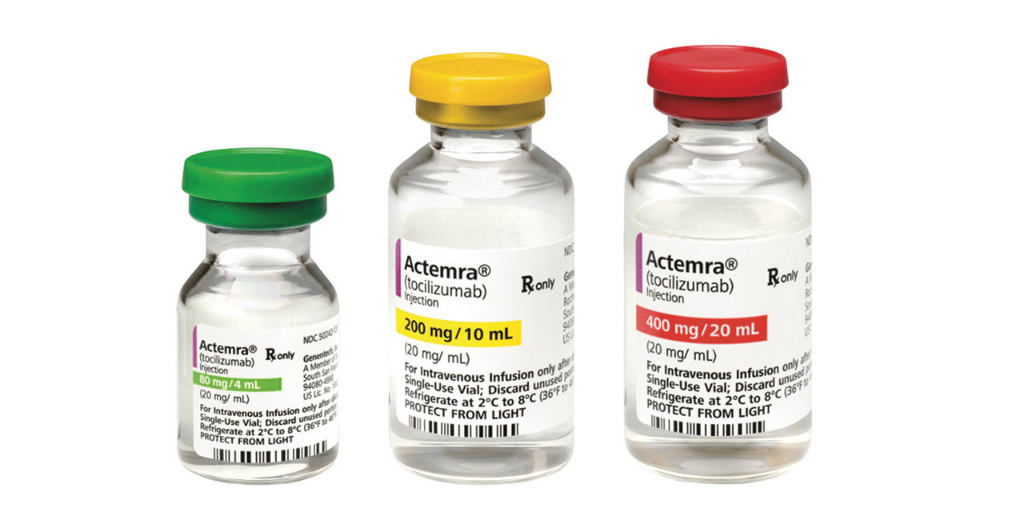
…Biopharmaceutical manufacturer Genentech notified 340B entities this week that it is running out of Actemra IV (tocilizumab)—a key tool in treating hospitalized COVID-19 patients. It told entities they will be subject to the same allocation procedures as other buyers.
The
…Passing a federal law that trims drug industry profits on companies’ top products by between 15% and 25% would lead to about 2 fewer drugs (-0.5%) being introduced in the U.S. in the law’s first decade, 23 fewer (-5%) in
…The House of Representatives passed a $3.5 trillion budget framework bill on Tuesday that potentially clears the path for Democrats to make extensive changes to U.S. health care delivery, although the impact on the 340B program remains to be seen.
…