Four major drugmakers recently urged a federal appeals court to reconsider its August ruling that allows a lawsuit—which alleges they [...] …
Articles by : Shannon Young, Associate Editor/Senior Writer
An oncology-focused biotech company recently announced it will soon set new limits on where it ships 340B-priced products and require [...] …
340B program advocates recently took aim at a new Republican-requested Congressional Budget Office (CBO) report on the 340B program, arguing [...] …
A controversial GOP-backed plan to overhaul the 340B program continued to draw pushback from provider advocates—as well as muted or [...] …
A U.K.-based drugmaker recently announced it will soon exempt covered entities in two additional states from its 340B contract pharmacy [...] …
A federal appeals court recently heard arguments in a case, which a group of Texas hospitals filed challenging a Department [...] …
Four major drugmakers recently announced refunds to 340B covered entities that purchased certain medications between 2022 and 2024, following recalculations [...] …
Breaking News
Carter, Harshbarger Reintroduce Controversial 340B ACCESS Act in U.S. House—This Time Without a Key Supporter
Two key U.S. House Republicans and vocal 340B program critics on Wednesday reintroduced controversial 340B overhaul legislation—but unlike last year’s [...] …
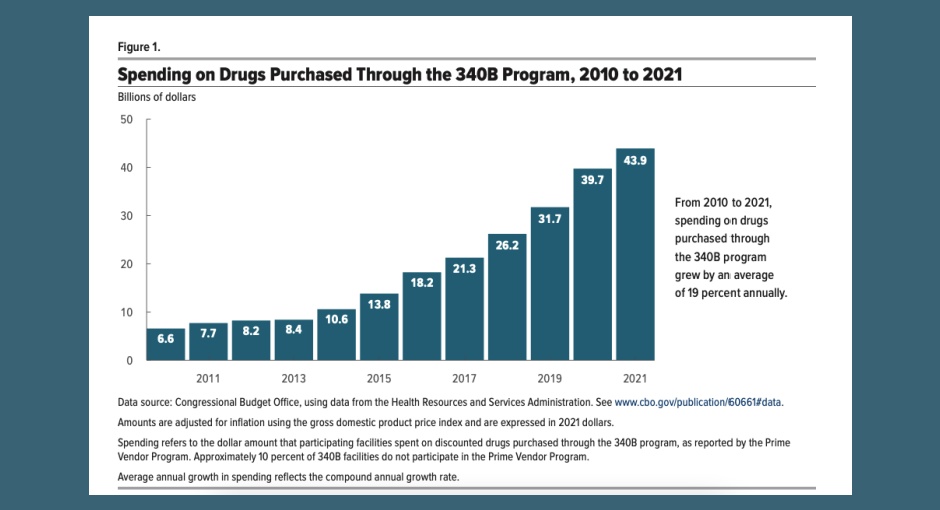
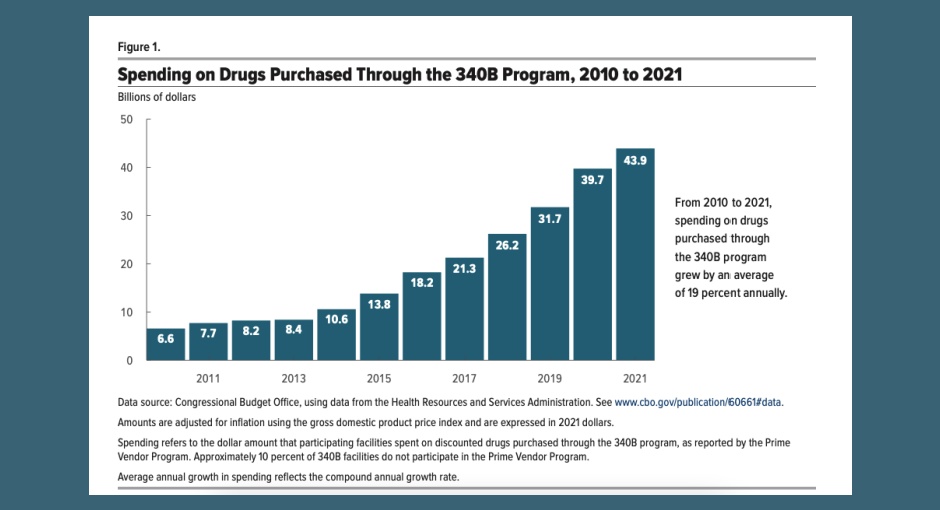
340B covered entities in the prime vendor program (PVP) spent nearly $44 billion on 340B drugs in 2021—up from $6.6 [...] …
A national hospital trade group urged federal officials Monday to investigate whether five major drugmakers engaged in anticompetitive practices while [...] …