Prescription drug sales at 340B prices grew 12.2% year-on-year from 2021 to 2022, drug industry consulting and research firm IQVIA says in a new white paper. That is down 3.7 percentage points compared with 340B sales growth from 2020
…Articles by : Tom Mirga, Editor at Large
Starting May 15, biopharmaceutical manufacturer Exelixis will require 340B covered entities that want past drug purchases that were not made at the 340B ceiling price to be reclassified as 340B sales to do so within 45 days of the date
…A U.S. Senate committee is attempting to mark up bipartisan legislation today to reform the pharmacy benefit management industry. The draft bill itself does not address the 340B program. Republican Sen. Mike Braun (Ind.), however, has filed an amendment for
…A federal district judge last week declined to make federal health officials give a South Carolina health center any more documents linked to the center’s brief expulsion from and subsequent reinstatement to the 340B program in 2018.
U.S. Chief District
…Research published online yesterday in the journal Health Affairs suggests that 340B hospitals are less likely than others to use biosimilar drugs, “possibly as a result of financial incentives making reference drugs more profitable than biosimilar medications.”
Groups that represent
…Drug manufacturer Bayer this morning significantly tightened its limits on 340B pricing when hospital covered entities contract with pharmacies to dispense drugs to patients. Grantee entities remain exempt from Bayer’s restrictions.
Covered entities reported receiving letters from Bayer about
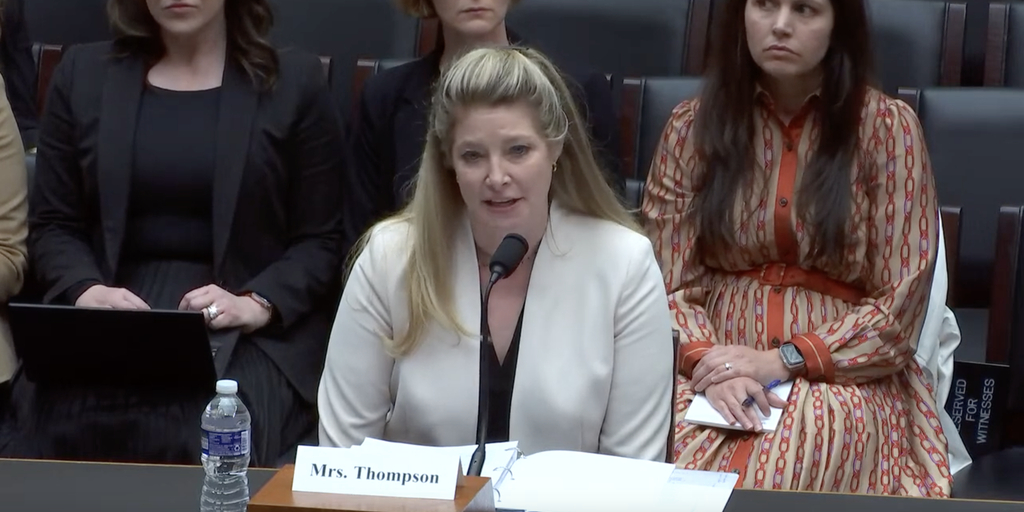
…An American Hospital Association senior executive told a U.S. House subcommittee yesterday her group opposes creating “significant new reporting requirements for 340B hospitals that do not reflect the true value or intent of the 340B program.”
But when asked by
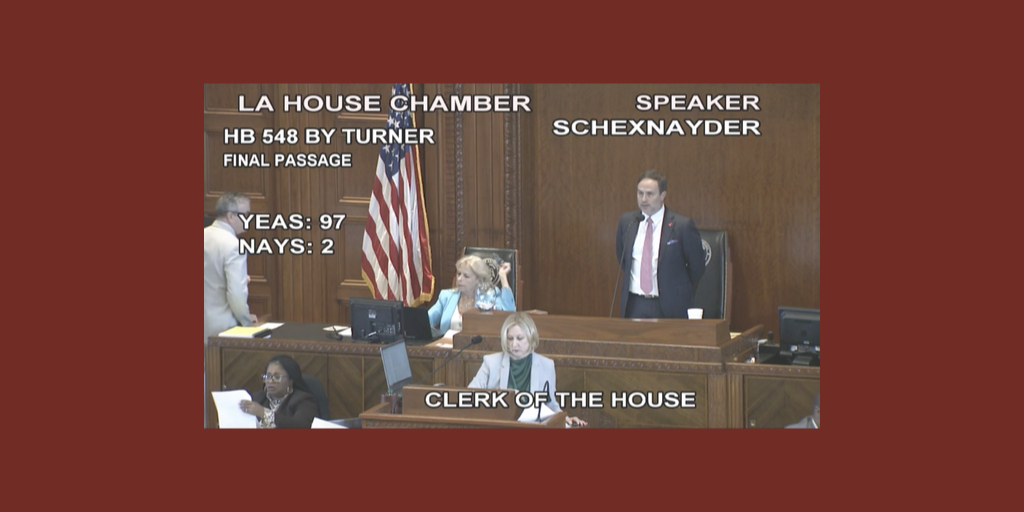
…Louisiana Takes Major Step Towards Prohibiting Drug Manufacturer 340B Contract Pharmacy Restrictions
The Louisiana House voted 97-2 yesterday to pass and send to the Senate legislation to stop drug manufacturer interference with 340B contract pharmacy arrangements. It also would prohibit pharmacy benefit manager and payor practices that lower reimbursement for 340B-purchased drugs.
…With so many advocacy groups using the term “340B” in their name these days, it’s easy to confuse one with another. Ted Slafsky’s latest column for Verity Solutions is a guide to the menagerie.
Slafsky, publisher and CEO of 340B
…This year’s annual 340B recertification period for U.S. Centers for Disease Prevention and Control grantees—Title X family planning, sexually transmitted disease, and tuberculosis clinics—will be May 8 through June 5, the U.S. Health Resources and Services Administration said Tuesday.
Recertification
…