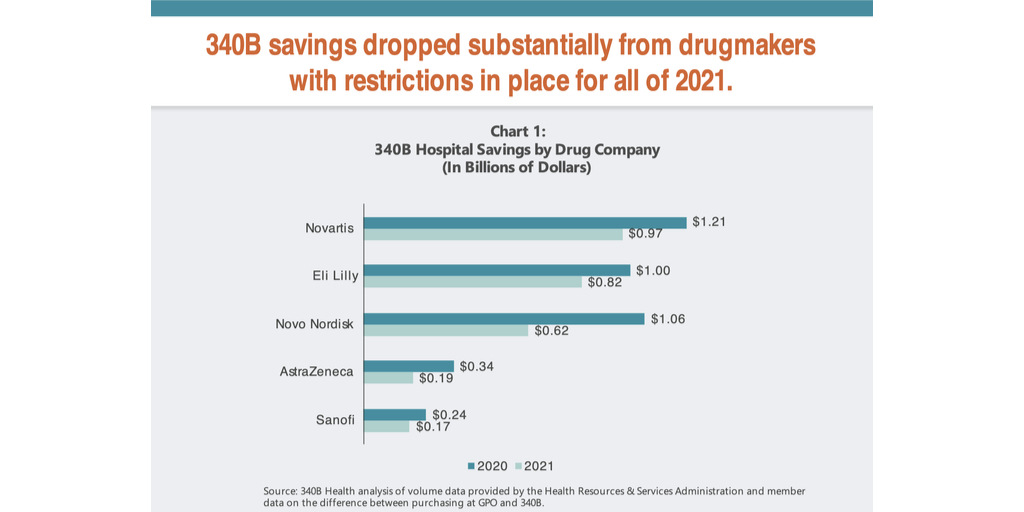
Hospitals’ 340B savings for the first five of the 21 companies to impose conditions on 340B pricing when providers use contract pharmacies decreased by an estimated $1.1 billion from 2020 to 2021, hospital group 340B Health said in a new
…Articles by : Tom Mirga, Editor at Large
Sean Dickson, a drug policy expert who has published frequently cited research on the 340B program and advised both health care providers and drug manufacturers on 340B compliance, has gone to work for the health insurance industry.
Dickson, most recently
…The fallout continues from drug makers and health centers’ decision to work together to change the 340B program, with the drug industry giving its first interview about the deal to 340B Report on Friday and several hospital groups jointly blasting
…Connecticut Gov. Ned Lamont’s novel bill to require hospital reporting on 340B revenue, prohibit drug maker restrictions on 340B contract pharmacy use, and prohibit pharmacy benefit manager discrimination against 340B entities and pharmacies has passed its first test in the
…The second quarterly period this year for 340B registration of new covered entities, outpatient facilities, and contract pharmacies will begin Saturday, April 1, and is expected to end on Monday, April 17.
Normally, a quarterly 340B registration period runs from
…Biopharmaceutical manufacturer Amgen this afternoon significantly expanded its conditions on 340B pricing when hospitals use contract pharmacies.
Johnson & Johnson announced a similar expansion of its 340B contract pharmacy limits last month. Hospital groups slammed J&J’s new restrictions.
Amgen’s
…U.S. House Republicans yesterday asked Department of Health and Human Services Secretary Xavier Becerra to testify March 29 about the Biden administration’s health care budget request.
Becerra will appear before the Energy & Commerce health subcommittee the day after it
…Congressional Medicare advisers yesterday formally recommended replacing the formula Medicare uses to distribute extra payments to safety net hospitals that disproportionately serve low-income and/or uninsured beneficiaries.
The 340B program uses the same calculation—the Medicare disproportionate share adjustment percentage—to determine eligibility
…The U.S. Centers for Medicare & Medicaid Services Tuesday announced the first 27 prescription drugs that will be subject to Medicare Part B inflation rebates, a list that includes AbbVie’s blockbuster arthritis drug Humira, which generated $5.6 billion in global
…The U.S. Health Resources and Services Administration has asked Congress for general rulemaking authority over the 340B program so it can establish “reporting requirements and definitions for the use of [340B] savings and contract pharmacy utilization.”
The Biden administration made
…