As we were the first to report on Tuesday morning, the Biden administration has proposed major changes to the 340B program administrative dispute resolution process, including stripping the Centers for Medicare & Medicaid Services and the Department of Health and
…Articles by : Tom Mirga, Editor at Large
Groups that represent 340B covered entities said they looked forward to studying the federal government’s proposal Tuesday to revamp the 340B administrative dispute process. But they expressed concern that drug manufacturers still are not being punished for denying 340B pricing
…Separating the 340B drug discount program into one for hospitals and another for non-hospital healthcare providers would be the wrong response to drug company denials of 340B pricing when providers use contract pharmacies, AIDS Healthcare Foundation said yesterday.
“A new
…The U.S. Health Resources and Services Administration this morning proposed a replacement for its two-year-old 340B administrative dispute resolution process. Comments are due Jan. 30.
“HRSA has encountered policy and operational challenges with implementation of the
2020 final rule,” the
…The U.S. Food and Drug Administration (FDA) last week approved the first gene therapy to treat adults with hemophilia B, a genetic bleeding disorder resulting from missing or insufficient levels of a protein needed to make blood clot. About 15%
…340B hospitals use biosimilar drugs less than other hospitals, which may expose patients to higher out-of-pocket costs, a drug industry funded study concludes.
Pharmaceutical Research and Manufacturers of America released the study last week. Healthcare consulting firm Milliman did the research for
…AIDS Healthcare Foundation sued Apexus, the federally contracted 340B prime vendor, on Friday for allegedly failing and refusing to negotiate sub-ceiling 340B discounts on HIV/AIDS prescription drugs pursuant to its agreement with the government.
An Apexus spokesperson yesterday said it
…The U.S. Health Resources and Services Administration has ordered Pennsylvania-based generic drug manufacturer KVK Tech to repay 340B covered entities for program violations found during an audit.
HRSA posted its audit findings Nov. 15. “KVK-Tech failed to refund covered entities for
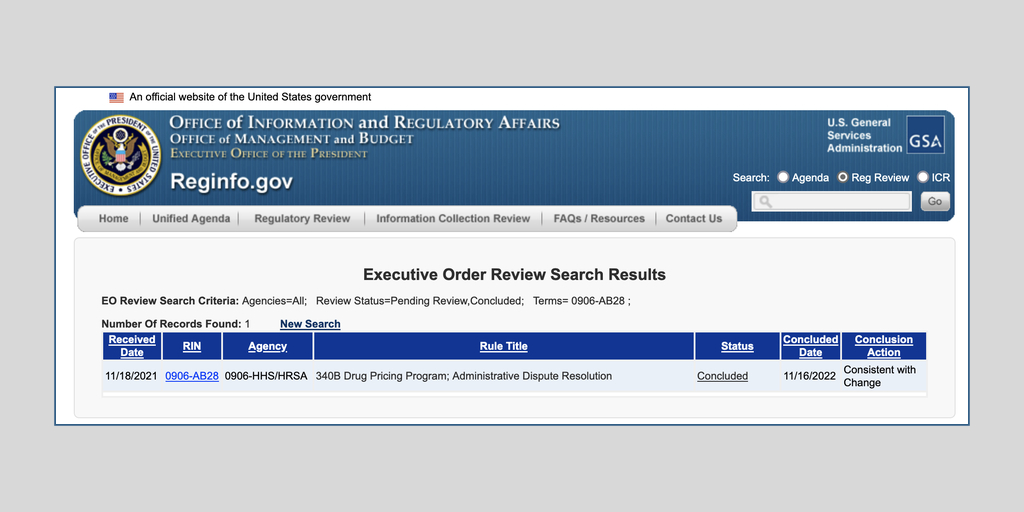
…The White House yesterday unexpectedly gave the U.S. Health Resources and Services Administration permission to publish a proposed replacement for HRSA’s December 2020 340B administrative dispute resolution final rule.
The Office of Management and Budget announced on its website Nov.
…