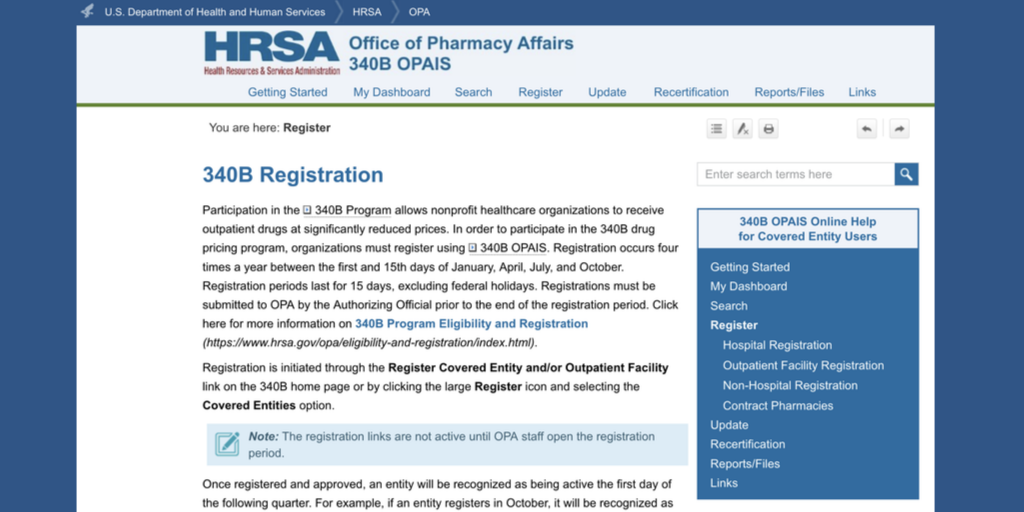
The third quarterly period this year for 340B registration of new covered entities, outpatient facilities, and contract pharmacies will begin tomorrow, July 1, and end on Friday, July 15.
Eligible hospitals, health centers, clinics, and their child sites and contract
…