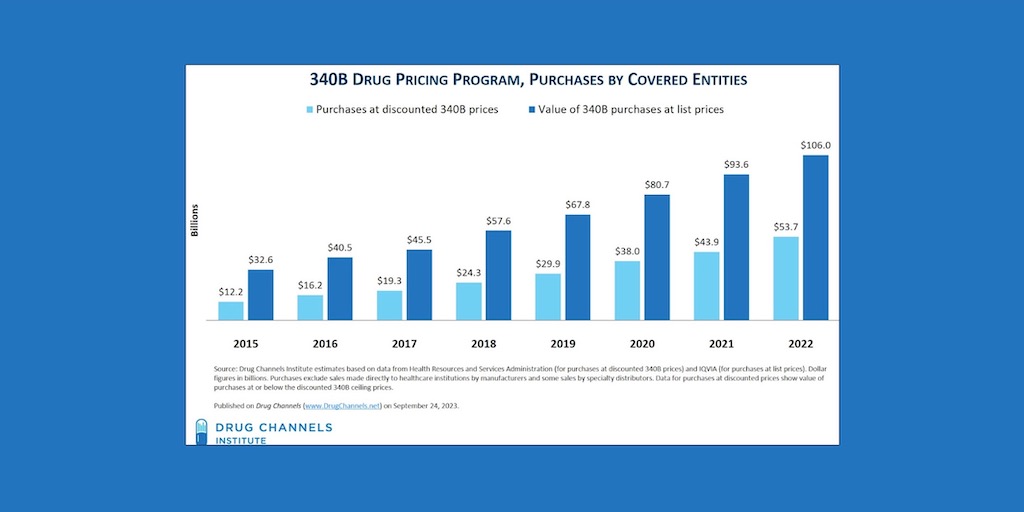
Drug purchases under the 340B program totaled $53.7 billion in 2022, or 22.3% more than the $43.9 billion in 2021, [...] …
Articles by : Tom Mirga, Editor at Large
There is no evidence to support federal allegations that a South Carolina community health center illegally diverts 340B-purchased drugs to [...] …
Novo Nordisk will issue refunds to 340B covered entities for some purchases this year of its blockbuster injectable diabetes medication [...] …
Eligible covered entities in Georgia may enroll in the 340B program immediately, rather than having to wait for the next [...] …
Drug manufacturer Sanofi recently started denying access for some health centers to 340B pricing at contract pharmacies after previously saying [...] …
Drug manufacturer Merck recently announced that it owes refunds to 340B covered entities for purchases of 17 products during the [...] …
Inaccurate points were ascribed to 340B advisers and service providers in a recent legal filing by four drugmakers as part [...] …
Drug manufacturer Janssen Biotech recently determined that it owes or may owe refunds to some 340B covered entities. The refunds [...] …
Drug manufacturer Tolmar recently said it plans refunds to 340B covered entities for overcharges on seven generic dermatologic products purchased [...] …
The Health Resources and Services Administration (HRSA) has awarded a $25.3 million, five-year contract to The Bizzell Group to audit [...] …