Drug manufacturer Boehringer Ingelheim announced this morning that, starting Aug. 1, all covered entity types will be subject to its conditions on 340B pricing when entities use contract pharmacies to dispense BI products. Until now, its conditions have applied to
…Articles by : Tom Mirga, Editor at Large
Federal health care officials can’t make a South Carolina health center obey their reading of the 340B patient definition for the same reason a federal appeals court said in January that the officials can’t force drug manufacturers to deliver 340B
…Louisiana Gov. John Bel Edwards (D) has signed legislation making his state just the second with a law addressing manufacturer conditions on deliveries of 340B-acquired drugs to contract pharmacies.
Arkansas’s Act 1103, passed in August 2021, was the first. A
…The six U.S. senators who asked Friday morning for ideas on ways to improve the 340B program elaborated on their reasons why in a news release that afternoon.
Sens. John Thune (R-S.D.), Debbie Stabenow (D-Mich.), Shelley Moore Capito (R-W.Va.), Tammy
…Ryan White Clinics for 340B Access yesterday criticized drug manufacturer Gilead Sciences for sponsoring a study that said it is unclear if patients of Ryan White clinics and other 340B grantee covered entities benefit from the drug margin these entities
…The U.S. Health Resources and Services Administration has added two days to the next quarterly 340B registration period.
A HRSA spokesperson said Friday that Q3 registration for 340B covered entities, their child sites, and their contract pharmacies will begin July
…News Alert
Six Democratic and Republican U.S. Senators Ask 340B Stakeholders for “Bipartisan Policy Solutions”
Six Democratic and Republican U.S. senators this morning asked 340B drug pricing program stakeholders for input on “bipartisan policy solutions that would ensure the program has stability and oversight to continue to achieve its original intention of serving eligible patients.”
…U.S. House Energy & Commerce Committee Chair Cathy McMorris Rodgers (R-Wash.) and Senate Finance Committee ranking Republican Mike Crapo (Idaho) are soliciting public input on what’s driving the increase in drug shortages, including whether or not the 340B drug pricing
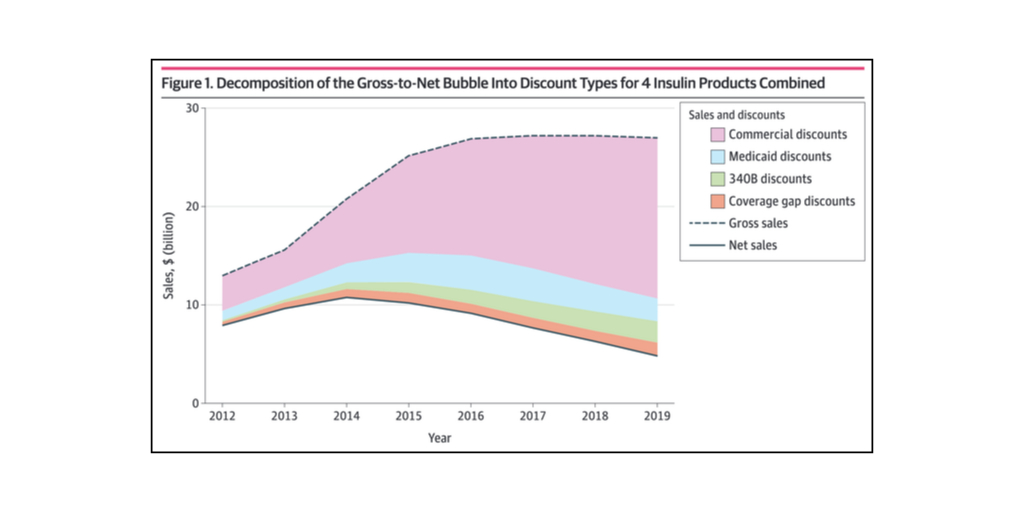
…Drug manufacturers often observe that a growing share of their take from gross sales goes to middlemen and others in the form of rebates and discounts. A term has been coined to describe the phenomenon: the gross-to-net bubble (the difference
…