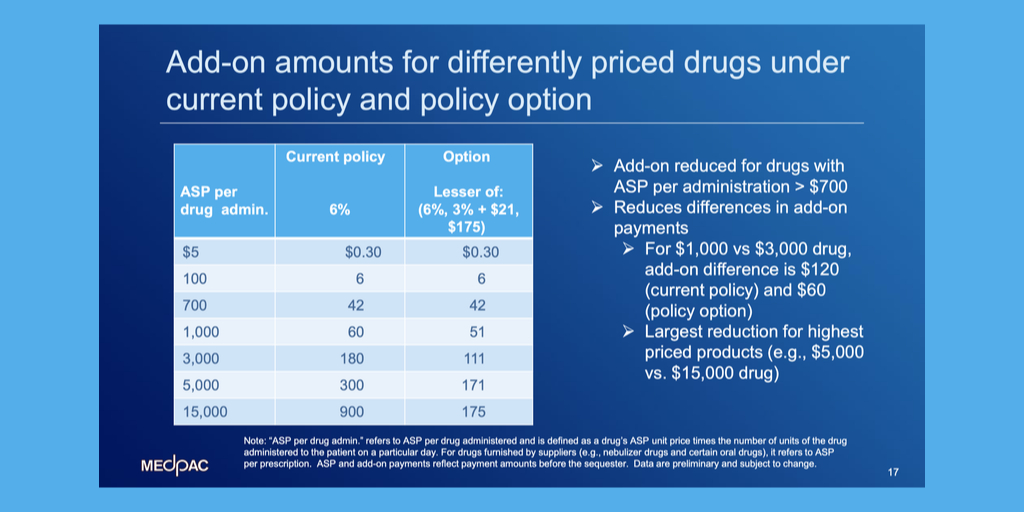
Today is the deadline for public comments on Medicare’s proposed rule for how it will pay for hospital outpatient services and clinician-administered drugs in 2023.
340B stakeholders will be paying close attention to comments on how Medicare Part B should
…