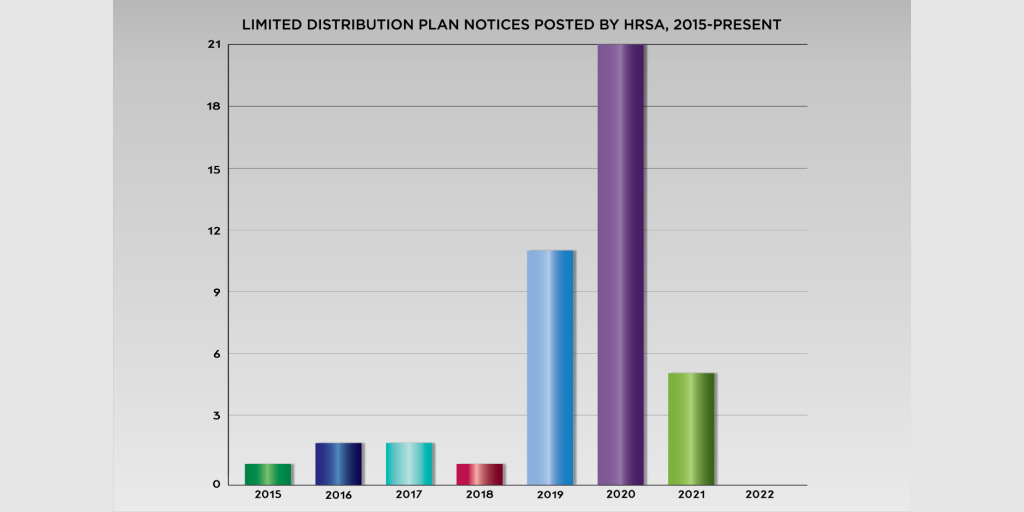
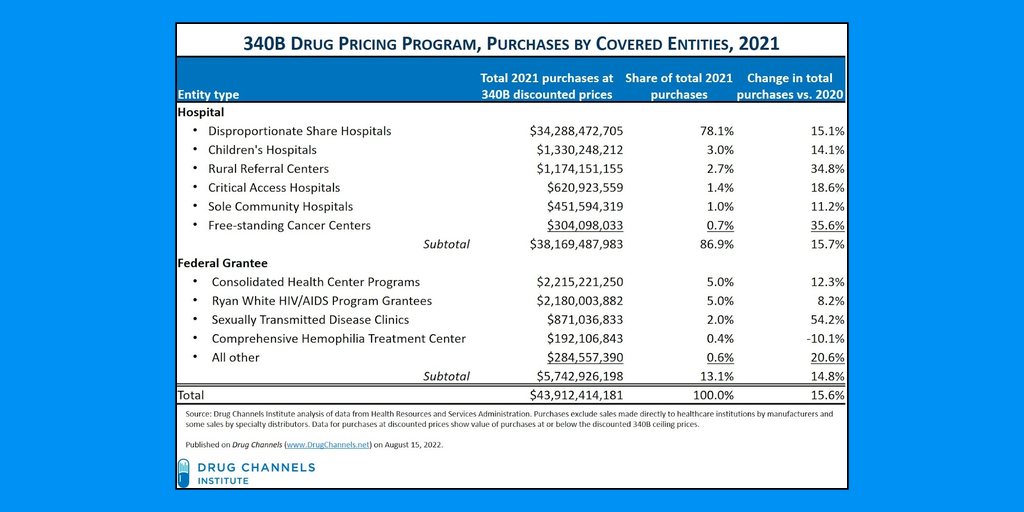
Last September, the U.S. Health Resources and Services Administration (HRSA) published a drug manufacturers’ notice to 340B covered entities that it was limiting distribution of a medicine that alleviates a side effect of HIV/AIDS treatment.
It was the fifth limited
…