The U.S. Health Resources and Services Administration’s (HRSA) proposed replacement for its year-old 340B administrative dispute resolution (ADR) process has been under White House review for almost 100 days—nearly a month longer that the anticipated release date announced in November.
…Articles by : Tom Mirga, Editor at Large
The U.S. Health Resources and Services Administration’s (HRSA) recent statement that only three hospitals so far have said on a federal form that they lost their 340B eligibility due to fallout from COVID-19 does not reflect the problem’s much bigger
…The National Rural Health Association (NRHA) asked U.S. Health and Human Services (HHS) Secretary Xavier Becerra this week to act against drug manufacturers that deny 340B pricing when covered entities use contract pharmacies.
NRHA CEO Alan Morgan also urged Becerra
…New research published in JAMA Network Open “raises doubt” about a major U.S. Centers for Medicare & Medicaid Services (CMS) justification for slashing hospitals’ Medicare Part B reimbursement for drugs purchased through the 340B program by almost one-third, the study’s
…A federal district judge in Florida has denied a group of defendants’ motion to dismiss Gilead’s lawsuit against them alleging fraudulent 340B-related transactions on the company’s HIV pre-exposure prophylaxis drugs Truvada and Descovy.
U.S. District Judge Aileen Cannon of the
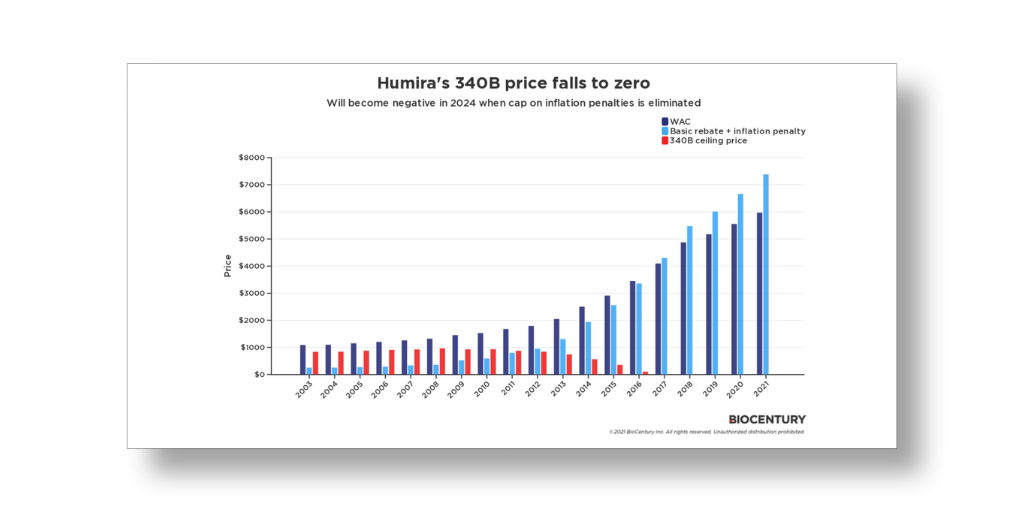
…In less than two years, brand drug companies that now must sell their products to 340B covered entities for a penny due to a history of price increases above inflation may have to start paying entities for their drugs instead
…UPDATED Thursday, Feb. 17, 2022, 4:00 p.m. EDT—The U.S. Health Resources and Services Administration (HRSA) said this afternoon, “The 340B program is an essential component of the safety net system that helps make health care and prescription drugs more affordable
…Insulin and diabetes drug manufacturers charged with federal and state price-fixing violations over their 340B contract pharmacy restrictions have again told a federal judge why they think she should dismiss the case.
AstraZeneca, Lilly, Novo Nordisk, and Sanofi filed their
…Drug manufacturer Merck is providing 340B covered entities with refunds for overcharges on 17 NDCs for purchases between Jan. 1 and March 31, 2019, the company says in a new public notice on the U.S. Health Resources and Services
…