A Florida state lawmaker told 340B Report this week his newly introduced Medicaid reimbursement bill will not affect how 340B [...] …
Articles by : William Newton, Associate Editor/Senior Writer
Breaking News
In Four Major Votes, Utah, South Dakota, Hawaii and Nebraska Each Advance 340B Contract Pharmacy Access Bills
Editor’s note: An earlier version of the headline for this article incorrectly stated that North Dakota advanced its contract pharmacy [...] …
News Alert
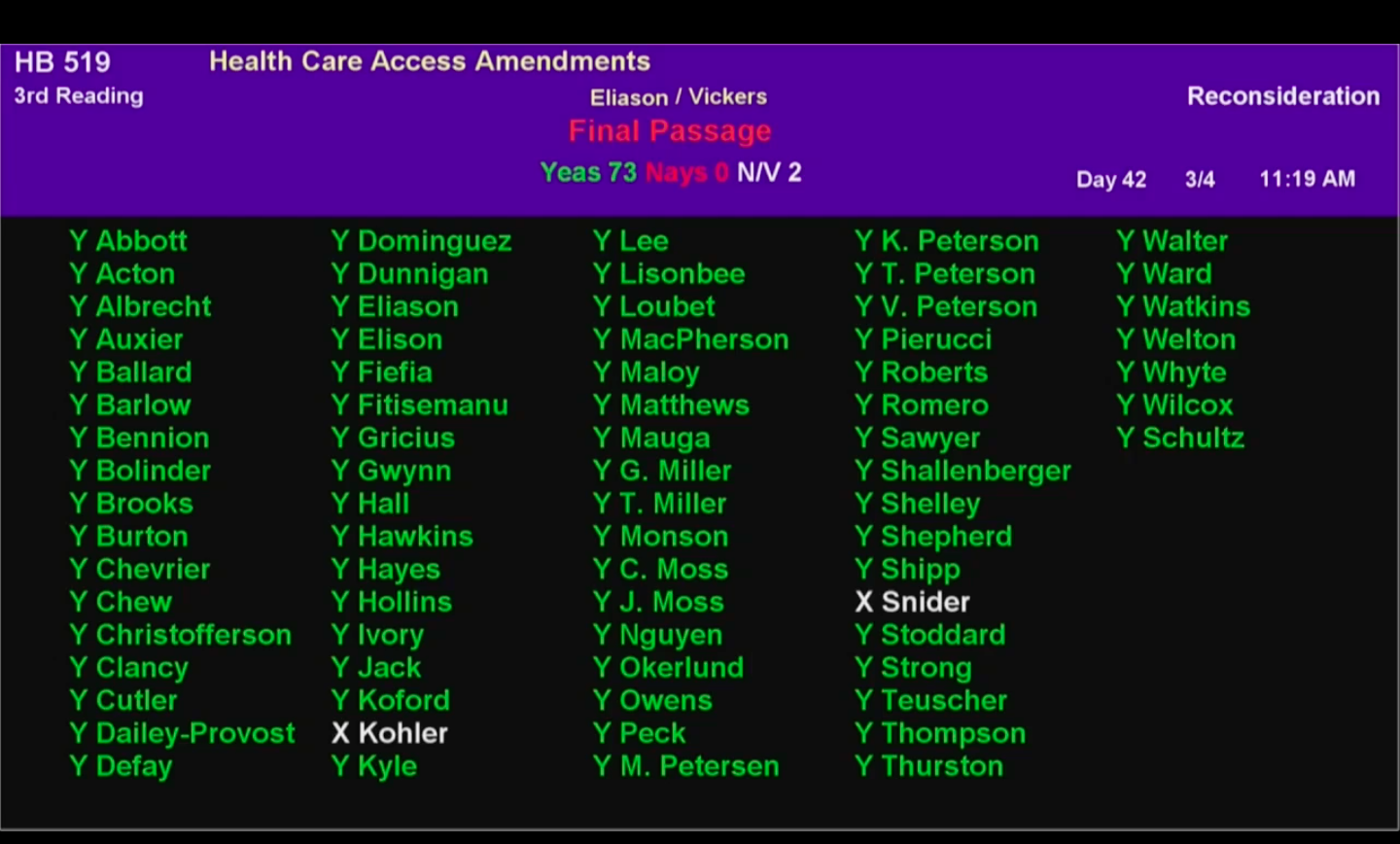
Utah House Unanimously Passes 340B Contract Pharmacy Access Bill, But Senate Pushes for Changes
The Utah House earlier today unanimously passed a bill to block drugmakers from restricting 340B contract pharmacy access in the [...] …
State lawmakers in Utah, New Mexico, Michigan, Kansas and Florida have all recently voted on or introduced bills to prohibit [...] …
Florida providers are concerned that a new state bill, which aims to lower Medicaid prices in the state, could significantly [...] …
The drug industry’s legal fight against Missouri’s 340B contract pharmacy access law was dealt another blow yesterday after a federal [...] …
Texas and Iowa have joined the growing list of states to introduce 340B contract pharmacy access bills in 2025, while [...] …
The University of Kansas Hospital (UKHA) this week sued the federal government for authorizing Johnson & Johnson (J&J) to audit [...] …
A federal judge yesterday rejected Swiss pharmaceutical company Novartis’ bid to block Missouri’s law prohibiting drugmaker 340B contract pharmacy restrictions [...] …
California lawmakers have introduced a 340B contract pharmacy access bill; Michigan legislators introduced a contract pharmacy bill that also includes [...] …