News Alert
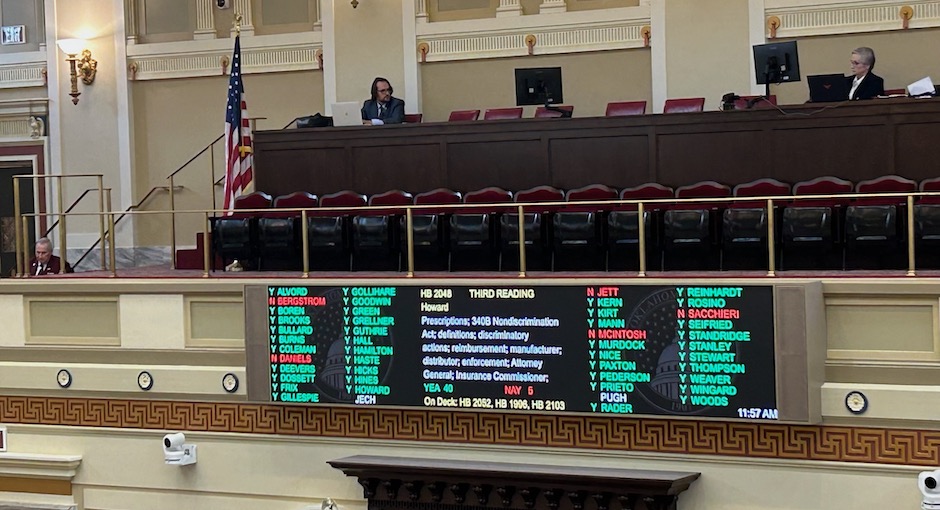
Rhode Island Senate Passes 340B Contract Pharmacy Access Bill, Vermont Vote Expected Today
The Rhode Island Senate late yesterday unanimously passed a bill to prohibit drugmaker 340B contract pharmacy restrictions, and Vermont state [...] …