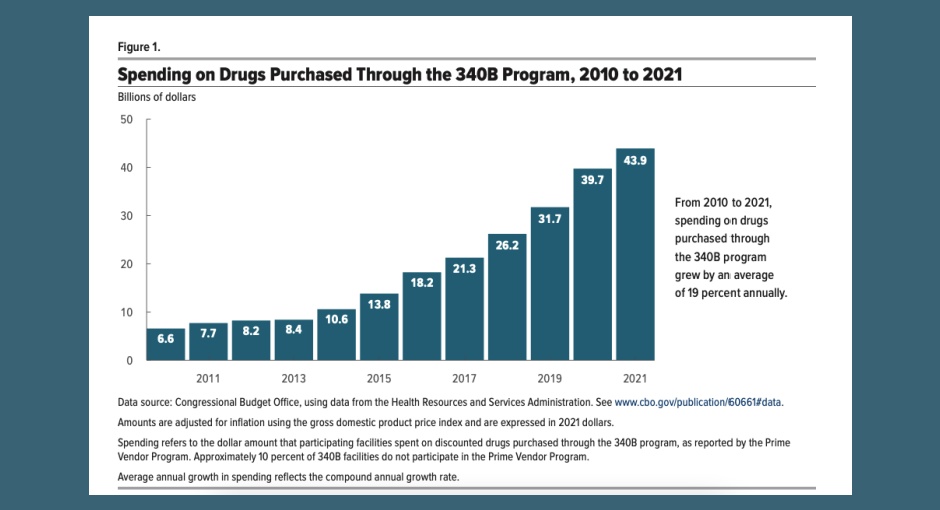
Blog Posts
SPONSORED CONTENT
Federally Qualified Health Centers (FQHCs) play a critical role in delivering accessible and affordable healthcare to underserved communities. However, they are facing mounting challenges that threaten their ability to maintain operations and meet growing community needs. With limited infrastructure, evolving regulations, upcoming Medicaid cuts, drops in the insured
…Breaking News
Federal Appeals Court Unanimously Upholds Mississippi’s 340B Contract Pharmacy Access Law Against AbbVie Challenge
SPONSORED CONTENT
Consider the following scenario: A recent change in electronic health record billing at one of your outpatient clinics has led to accumulation errors in your 340B split-billing software, resulting in overpurchasing of 340B drugs. Although hypothetical, this scenario illustrates just one of many inventory-related situations
…