Part 5 of 6—Revlimid “didn’t get any better…. You just got better at making money”
Last September, the Democratic-controlled U.S. House Oversight and Reform Committee released a 45-page report focused on Celgene and Bristol Myers Squibb’s (BMS’s) nearly two dozen price increases on Revlimid from 2005, when Celgene bought the drug, through 2019. It was part of a two-year committee investigation of several leading drug manufacturers’ price increases, executive compensation, and alleged strategies to limit competition and maximize profits.
House Oversight investigators did not look at Celgene/BMS’s compliance with 340B pricing obligations regarding Revlimid and its other two myeloma drugs, Pomalyst and Thalomid. The report, however, found that:
- Celgene/BMS raised Revlimid’s price 23 times between 2005 and 2019, from $215 per pill to $763 per pill—a 254 percent increase. (The cumulative U.S. inflation rate for urban consumers, CPI-U, for that same 14-year time span was 30.9 percent.)
- Celgene’s net U.S. revenue from Revlimid rose from $1 billion in 2009 to nearly $6.5 billion in 2018. This rise in revenue from Revlimid fueled Celgene’s annual profits, which increased from $780 million in 2009 to $4 billion in 2018, the report said.
- “Internal communications show that pricing decisions made by Celgene executives—including former CEO Mark Alles—were driven almost exclusively by the need to meet company revenue targets and shareholder earnings goals,” the report said. “Celgene’s price increases for Revlimid led directly to higher bonuses for its executives.”
- Celgene targeted the U.S. market for price increases while maintaining or cutting prices for the rest of the world.
- “Celgene suppressed competition by abusing” the U.S. Food and Drug Administration-directed Risk Evaluation and Mitigation Strategies (REMS) safety program for Revlimid, the report said, using its REMS to stall 14 generic versions of Revlimid from coming to market. (The whistleblower’s lawsuit alleged that Celgene/BMS excluded 340B covered entities from its limited distribution network for Revlimid, not because doing so would weaken its REMS for the drug, as the company claims, but because by doing so it avoids paying 340B ceiling prices on Revlimid. In a statement to 340B Report, BMS/Celgene) Mylan, the maker of one of the potential competing drugs, sued Celgene for access samples of Revlimid that it needed to obtain FDA approval but which Celgene refused to provide, citing its REMS. Celgene and Mylan settled the suit in July 2019, with Celgene paying Mylan $62 million, the congressional report said. On April 28, 2021, Celgene sued India-based generic drug manufacturer Hikma Pharmaceuticals for allegedly violating 11 of Celgene’s patents on Revlimid. Hikma is seeking FDA approval to import and market a generic version of Revlimid in the United States.
- Celgene/BMS’s price increases on Revlimid were not justified by past or future investment in research and development, nor by rebates, discounts, and other fees paid to pharmacy benefit managers.
U.S. Rep. Debbie Wasserman Schultz (D-Fla.) homed in on the Revlimid report’s allegations of REMS abuse during a Sept. 30, 2020 hearing at which former Celgene CEO and board chairman Mark Alles testified.
“Mr. Alles, did Celgene also view the REMS program as a way to delay generic entry?” Wasserman Schultz asked Alles. “We did not,” Alles answered. “We did offer to sell and would sell samples of our products to generics once they agreed to our common-sense safety standards and an indemnification process.”
Wasserman Schultz then referred to an internal Celgene document stating that one benefit of implementing a REMS program was the “prevention of generic encroachment.”
“Mr. Alles, prevention of generic encroachment just means to delay generic competition. There is just no other way to read that,” Wasserman Schultz said. “So, I will ask you again. Did Celgene use its REMS program to try to prevent generic competition, yes or no? And you are looking at a document that—whose definition, ‘prevention of generic encroachment,’ would seem to be exactly that.”
Alles answered “I see the slide, and I understand some of the words, but I don’t know the context. And what I can say…”
“Oh, come on. Come on,” Wasserman Schultz interjected.
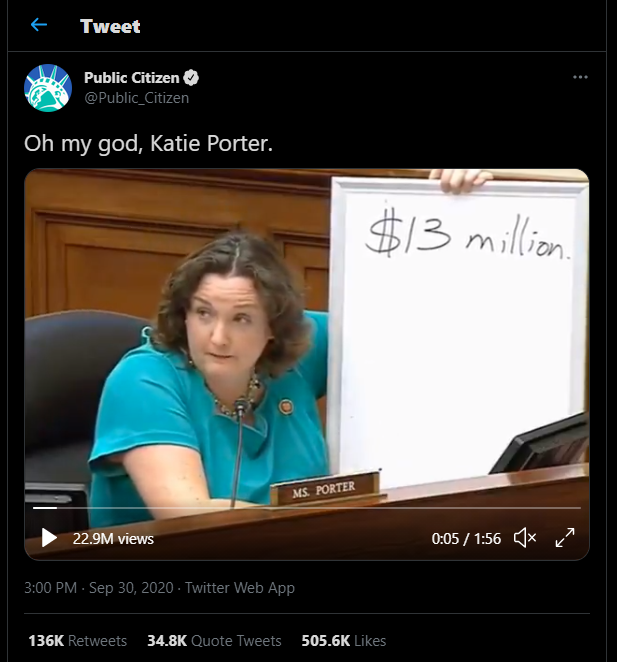
A video of Rep. Katie Porter (D-Calif.) questioning Alles during the hearing about Celgene’s Revlimid price increases and their connection to Alles’ pay has been viewed almost 23 million times on Twitter.
Regarding Revlimid’s price increases from 2005 through 2019, Porter rapidly asked Alles whether Revlimid became more effective in that time, whether patients needed fewer pills, if the drug began working faster and had fewer side effects, or if formulation or production changes justified a higher price. Then Porter asked Alles about the connection between Revlimid’s profitability and his compensation.
“Mr. Alles, do you know how much you personally received in bonuses over two years, the last two years, just because Celgene raised the price of this one drug, Revlimid?”
“I received very generous compensation, but I don’t know the exact number that you’re referring to,” Alles answered.
“In fact, you personally received $500,000 personally just by tripling the price of Revlimid. So, to recap here, the drug didn’t get any better. The cancer patients didn’t get any better. You just got better at making money. You just refined your skills at price gouging.”
In his written testimony, Alles said Celgene’s pricing decisions for its medicines “were guided by a set of long-held principles that reflected our commitment to patient access, the value of a medicine to patients and the health care system, the continuous effort to discover new medicines and new uses for existing medicines, and the need for financial flexibility.”
Current BMS Chairman and CEO Giovanni Caforio, who also was called to testify at the September 2020 hearing, said in his written statement that “Revlimid’s REMS program ensures that doctors, patients, pharmacists, and others are handling Revlimid with the care that it warrants.”
Caforio said BMS bases it drug prices on factors including “the value of scientific innovation for patients and society in the context of overall healthcare spending; economic factors in relation to the healthcare systems’ capacity to provide appropriate, rapid, and sustainable access to patients; and the ability to sustain our research and development investment in new innovations that address serious unmet medical needs.”
“With a product like Revlimid, which is extending the lives of patients with serious blood cancers, the value to patients is truly immeasurable,” he said.
340B Report asked BMS/Celgene whether it concurs with the committee’s findings related to price increases and whether it had any additional reactions or comments regarding the committee report. It declined to address either matter but did provide the following statement prior to the publication of our series.
Have you had trouble accessing 340B pricing for Revlimid and other BMS/Celgene myeloma drugs? Do you have other stories that we should be covering? Reach out to us at tom.mirga@340breport.com.
Have you found this investigative series and our other reporting to be important? Please pass the word to your colleagues outside of your organization and let them know about our lowest-ever discount offer: 25% off our regular subscription rates, only available through June 11. They can subscribe now using coupon code INVESTIGATE25. For questions, please contact reshma.eggleston@340breport.com.
Monday in Part 6: What happens next for Revlimid, Pomalyst, Thalomid, and 340B?


