Pennsylvania health centers are upset about what they say was the state Medicaid office’s disclosure without warning last week that, starting July 1, it wants drug claims billed to Medicaid managed care organizations to include a modifier showing if the
…Articles by : Tom Mirga, Editor at Large
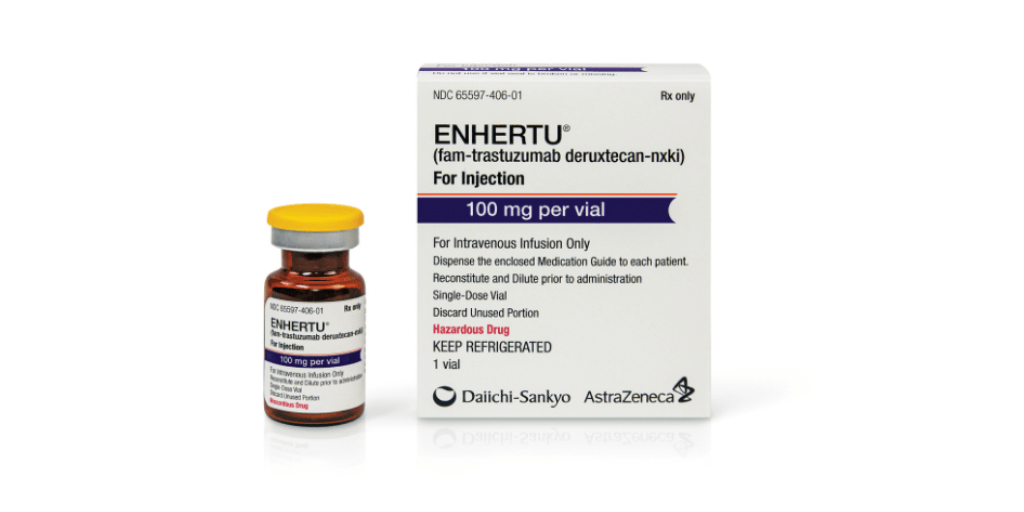
Drug manufacturer Daiichi-Sankyo is offering 340B covered entities refunds for overcharges on metastatic breast cancer treatment Enhertu during Q1 2020 and on cholesterol medication Welchol for Oral Suspension during Q1 2022 and Q4 2022.
The U.S. Health Resources and Services
…U.S. Rep. Larry Bucshon (R-Ind.), vice chair of the House Energy & Commerce health subcommittee, has written a bill to impose significant 340B program reporting requirements on disproportionate share hospitals and potentially other covered entities. Meanwhile, two bills have been
…U.S. Health Resources and Services Administration Administrator Carole Johnson said on Capitol Hill yesterday that HRSA would welcome congressional help ensuring there is “accountability and transparency” in the 340B program.
Johnson was answering questions from Rep. Morgan Griffith (R-Va.) during
…A Philadelphia-area hospital that closed last year after its sale fell through has filed a federal antitrust class action against healthcare conglomerate CVS over its 340B contract pharmacy policies.
The same shuttered hospital has been challenging in state court its
…The federal government yesterday decided not to ask all 14 judges on the U.S. Third Circuit Court of Appeals in Philadelphia to review a three-judge panel’s unanimous January opinion upholding AstraZeneca, Novo Nordisk, and Sanofi’s restrictions on 340B pricing when
…Drug manufacturer Eli Lilly asked a 340B covered entity last week to promptly repay it for “duplicate 340B/Medicaid discounts” and evaluate all Lilly prescriptions that it or its contract pharmacies dispensed for the last three years.
Consultants and attorneys with
…About a dozen people who advise, help run, or work at 340B covered entities are launching a nonprofit group, 340B Truth, to try to hammer out a covered entity consensus position on 340B reform and urge Congress to pass it
…The U.S. Department of Health and Human Services Office of Inspector General said yesterday it has no comment on the status of referrals from the U.S. Health Resources and Services Administration to fine eight drug manufacturers for failing to offer
…A California state appeals court has affirmed a lower state court’s judgement that a 340B contract pharmacy overcharged the state Medicaid agency, Medi-Cal, nearly $2.5 million more than the pharmacy was entitled to receive for 340B drugs between May 2015
…