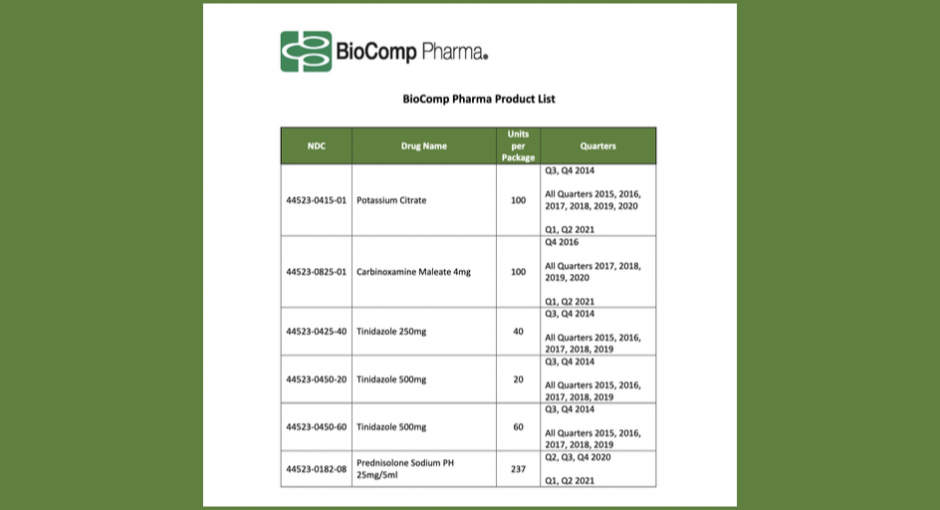
Generic-drug manufacturer BioComp is offering covered entities refunds for lower 340B prices that it did not pay on six NDCs for periods over eight years, starting in Q3 2013 and ending in Q2 2021.
BioComp, a wholly-owned subsidiary of San Antonio, TX-based Mission Pharmacal, announced the refunds on the U.S. Health Resources and Services Administration (HRSA) website last week. HRSA audited BioComp during fiscal 2021. The published audit findings said the company “did not offer covered outpatient drugs to eligible covered entities at the statutory ceiling price.”
In the notice on HRSA’s site, BioComp asked entities that bought any of the six products to fill out and return an attached refund request form by June 30. “BioComp will review and issue refunds as appropriate under the circumstances of each request for a refund,” the notice said. Entities also must provide their federal taxpayer identification number and proof of purchase.
“Not a Mere Oversight”
“That a company could get away with not offering 340B discounts for eight years highlights the need for HRSA to be auditing drug companies more frequently or at least spot-checking manufacturer ceiling price calculations on a more frequent basis,” Powers Law partner William von Oehsen said. “Failure to detect a violation for this duration is not a mere oversight. It suggests that the agency needs to take the problem of manufacturer non-compliance more seriously.”
HRSA audited five manufacturers in FY 2021. Four had adverse findings and were required to make repayments, including BioComp. HRSA audited 200 covered entities during the same period. Thirty-seven were required to make repayments due to 340B program infractions. HRSA conducts both targeted and random audits of manufacturers and entities.