SPONSORED CONTENT
The evolving landscape of pharmaceutical pricing and reimbursement models presents complex challenges for manufacturers and pharmacies. One of the most significant developments is the introduction of the Inflation Reduction Act (IRA) and its Maximum Fair Price (MFP) provisions, aimed at reducing drug costs for Medicare beneficiaries.
Beginning January 1, 2026, Medicare Part B plans will reimburse pharmacies at the MFP, a price negotiated between CMS and manufacturers. For covered entities, the MFP mandates only the lower of the 340B price or MFP can be claimed. The responsibility for ensuring non-duplication of the MFP has been left to manufacturers and CEs, as outlined in Section 40.4.5 of the Final Guidance.
A collaborative, neutral clearinghouse is critical in addressing the challenges posed by the IRA’s MFP provisions. The clearinghouse supports both CEs and manufacturers in navigating the complexities of the IRA’s MFP requirements, with emphasis on the 340B program.
What is the IRA and MFP?
The Inflation Reduction Act (IRA) is a transformative piece of legislation, signed into law on August 16, 2022. Key provisions include empowering Medicare to negotiate prices for certain high-cost drugs and capping annual out-of-pocket prescription costs for Medicare beneficiaries. The IRA also introduces drug price inflation rebates, ensuring manufacturers pay penalties if prices rise faster than inflation.
The MFP is a key provision of the IRA, designed to make prescription drugs more affordable for Medicare beneficiaries by authorizing Medicare to negotiate directly with manufacturers to establish fair prices for several high-cost drugs covered under Medicare Part B and D. MFP represents the ceiling price Medicare will pay for these drugs, ensuring costs are more predictable and affordable.
The MFP process will begin January 1, 2026. Manufacturers must comply with the negotiation process, and failure to do so can result in financial penalties. Pharmacies typically purchase drugs at the Wholesale Acquisition Cost (WAC), which can be significantly higher than the MFP, however they are reimbursed based on the MFP. This discrepancy creates a financial gap that manufacturers must reimburse.
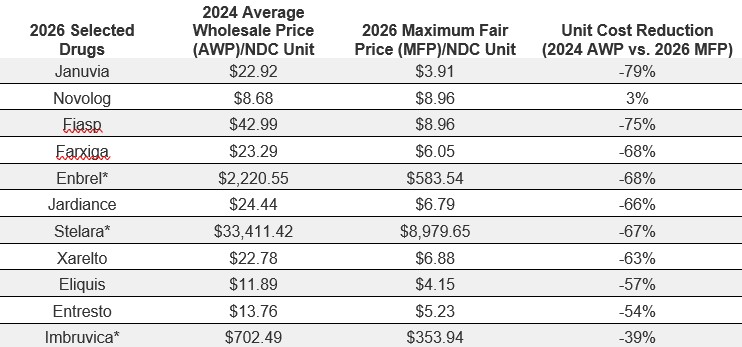
The Intersection of 340B Drug Pricing and MFP
The IRA’s drug price negotiation and inflation rebate measures have the potential to fundamentally reshape the pricing dynamics of drugs covered under the 340B program.
For pharmacies participating in 340B, manufacturers may be in a situation where it could be subject to having to provide both a 340B discount and MFP reimbursement for the same drug. This creates a “double discount” issue, like the Medicaid duplicate discount scenario, where manufacturers end up being subject to both Medicaid and 340B discounts on the same medication. It is important for there to be a mechanism trusted by all parties — providers, manufacturers and the government—to prevent this scenario. MFP may be lower or higher than the 340B price, and for the 10 drugs selected for 2026, both scenarios are observed. Manufacturers are required to provide the lower of the two prices. As shown in the table below, for covered entities, only in the case of Product B does it make financial sense to claim the drug under the 340B program when the claim is for Medicare.
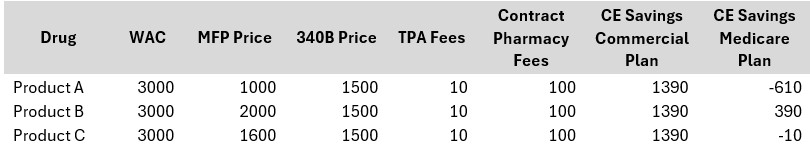
As more drugs are added to the MFP list annually, the potential for error increases, complicating the interaction between 340B program pricing and MFP provisions. To address this, CMS requires manufacturers, pharmacies, and CEs to have mechanisms in place to ensure timely reimbursement to pharmacies while applying both MFP and 340B discounts correctly.
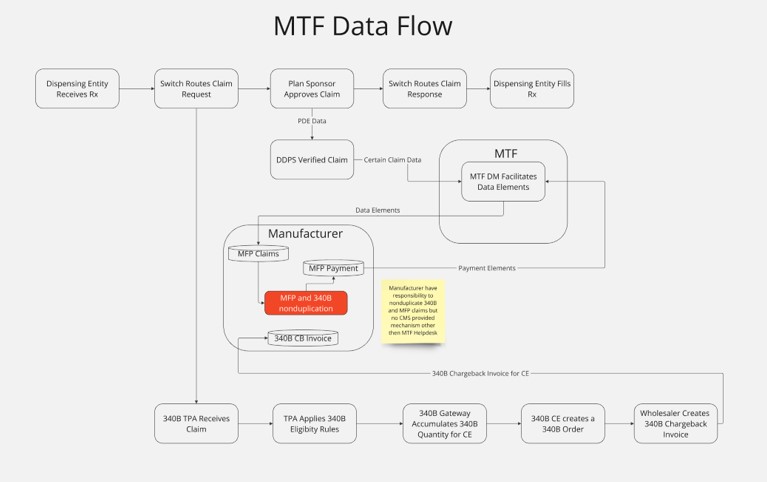
A Solution for the Future
A robust solution is needed to manage the intricate dynamics between the 340B program and MFP pricing. During the CMS comment period on the IRA MFP provisions, many CEs and organizations endorsed a clearinghouse as a critical mechanism for resolving the MFP/340B discount complexities.
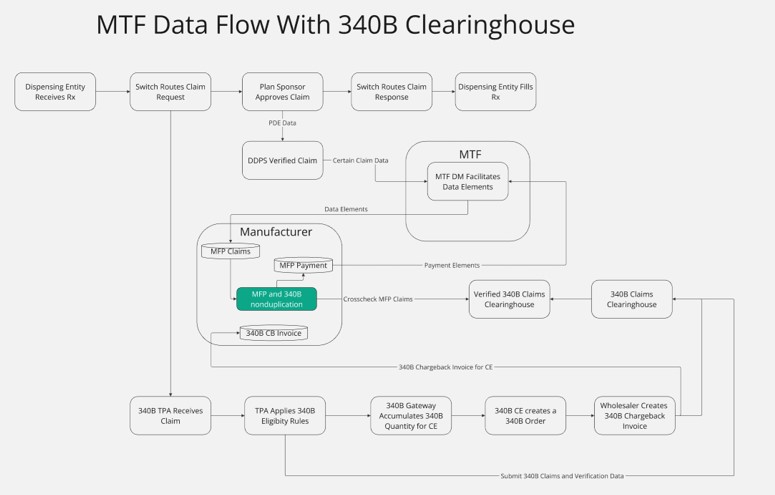
A clearinghouse serves as a centralized platform designed to address the needs of manufacturers, pharmacies, and covered entities. It facilitates the identification and resolution of pricing discrepancies, ensures accurate reimbursement, and improves the overall efficiency of the pharmaceutical supply chain.
This streamlined approach requires no changes to existing CE processes and relies solely on the same information currently provided to 340B vendors, ensuring minimal disruption while addressing the pricing challenges effectively. The clearinghouse promotes fair pricing practices while ensuring Medicare beneficiaries retain access to affordable medications.
Furthermore, the clearinghouse supports CEs in evaluating the most advantageous pricing option—whether MFP or 340B—empowering them to maximize savings, enhance operational efficiency, and ensure sustainability.
A Market-Ready Solution
RxParadigm offers a national clearinghouse solution designed to address the complexities of IRA and MFP pricing models. The platform provides a reliable and efficient system to help pharmacies, CEs, and manufacturers manage and resolve pricing discrepancies effectively.
The clearinghouse is fully equipped to handle issues such as rebate double payments, ensuring equitable solutions for both covered entities and drug manufacturers. It facilitates the resolution of challenges like duplicate discounts on the same transaction, supporting manufacturers in providing accurate reimbursements while safeguarding pharmacies against financial losses. The platform’s automated processes help reduce administrative workloads and maintain accurate pricing across the supply chain.
The clearinghouse enables covered entities to identify and resolve pricing discrepancies, such as instances where the MFP price exceeds the 340B price or vice versa. This functionality promotes transparency, supports data-driven decision-making, and ensures manufacturers avoid over-reimbursement while ensuring fair compensation for pharmacies dispensing the medications.
Through the advanced capabilities of the Tungsten+PLUS™ system, manufacturers, covered entities, and pharmacies are equipped to navigate the complexities of IRA and MFP pricing models effectively. This approach promotes transparency, fairness, and accountability, fostering stronger trust and collaboration among all stakeholders in the healthcare ecosystem.

Mesfin Tegenu is CEO and Chairman of RxParadigm, a technology solutions provider committed to supporting initiatives that advance the 340B program. He can be reached at Mesfin.Tegenu@rxparadigm.com.



