Editor’s Note: With 340B-related litigation heating up in several states following the enactment of new contract pharmacy access laws, 340B [...] …
Blog Posts
340B covered entities and drugmakers offered competing criticisms Thursday of the Health Resources and Services Administration’s (HRSA) newly released 340B [...] …
The Health Resources and Services Administration (HRSA) today unveiled its highly anticipated 340B rebate guidance, proposing a pilot program that [...] …
Three federal appeals judges—who have all ruled on 340B matters in the past—will hear oral arguments in September in a [...] …
A majority of 340B hospitals surveyed said the costs associated with implementing drugmakers’ proposed rebate models would hinder their ability [...] …
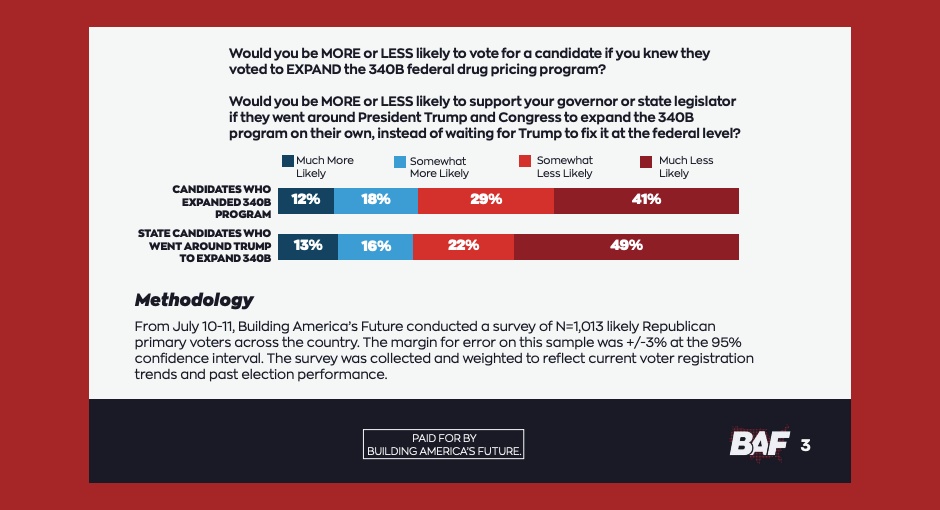
The dark money group behind a campaign targeting GOP-led states’ recent efforts to enact 340B contract pharmacy access laws released [...] …
SPONSORED CONTENT
The regulatory landscape for the 340B drug pricing program is evolving rapidly. As scrutiny from the Health Resources and Services Administration (HRSA) and drug manufacturers intensifies, healthcare leaders and 340B teams face growing pressure to maintain airtight
…
Three patient groups are urging a federal appeals court to back drugmaker 340B rebate models they argue would bring transparency [...] …
Two major drugmakers recently announced new states that will be exempt from their 340B contract pharmacy restrictions following their enactment [...] …
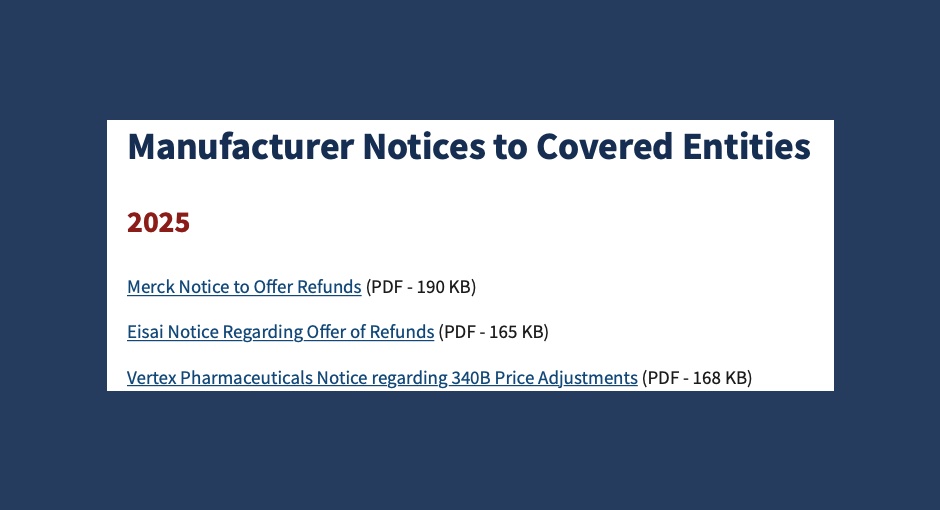
Three drugmakers recently announced refunds to 340B covered entities that purchased certain medications between 2022 and 2025 following recalculations of [...] …