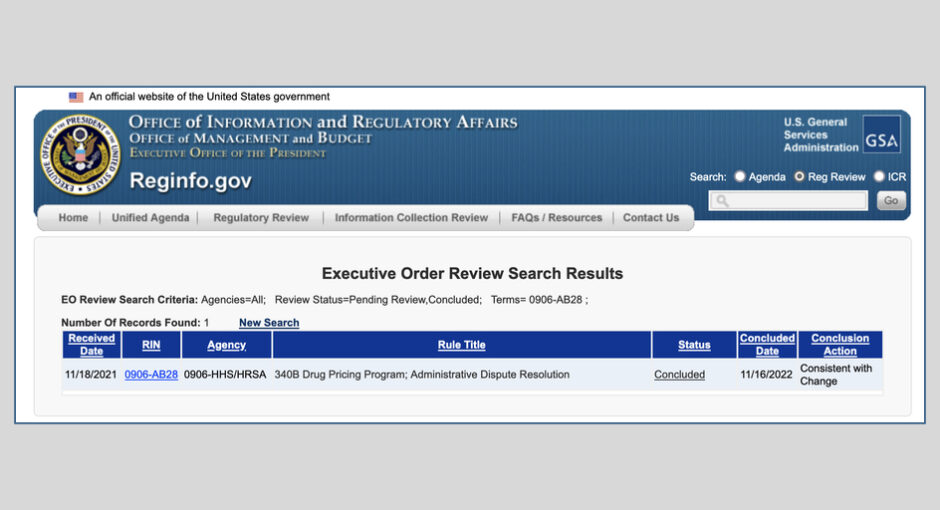
The White House yesterday unexpectedly gave the U.S. Health Resources and Services Administration permission to publish a proposed replacement for HRSA’s December 2020 340B administrative dispute resolution final rule.
The Office of Management and Budget announced on its website Nov. 16 that it concluded its review of HRSA’s ADR proposed rule. HRSA submitted the proposed rule to OMB for clearance to be published in the Federal Register almost exactly one year ago, on Nov. 18, 2021.
In December 2021, the Biden administration provided more details on its plans, announcing that it wanted to replace the ADR regulation that was enacted in the last days of the Trump administration with an entirely new rule that “better aligns with the president’s priorities on drug pricing [and] better reflects the current state of the 340B program.”
The contents of the proposed rule are unknown. It had not yet been posted for public inspection as of noon Eastern today. It could be released to the public as soon as later today.
In March 2021, a federal district judge in Indianapolis granted drug manufacturer Eli Lilly’s motion for a preliminary injunction stopping the HRSA from implementing or enforcing its current ADR regulations against the company. Drug manufacturer Sanofi and Pharmaceutical Research and Manufacturers of America are also challenging the legality and constitutionality of the existing ADR regulations, arguing, among other things, that the manufacturers were not given an opportunity to provide comments on the regulation. The government has argued that the manufacturers had an opportunity to comment when the regulation was originally proposed in 2017.
HRSA had said on the OMB website that its proposed replacement for its existing ADR rule “would establish new requirements and procedures for the 340B program’s ADR process. This administrative process would allow covered entities and manufacturers to file claims for specific compliance areas outlined in the statute after good faith efforts have been exhausted by the parties.”
Why it has taken this long for the proposed rule to be cleared for publication is a mystery. It originally was slated to be published in January 2022. Why it has been cleared now also is not known.
There is no statutory limit on how long OMB can take to review an agency regulation or guidance document. Under a Clinton administration executive order, the period for OMB review is limited to 90 days. The OMB director can extend the review period on a one-time basis for no more than 30 days. The review period also can be extended indefinitely by the head of the rulemaking agency.
340B Report previously asked both HRSA and OMB whether the Health and Human Services Department or HRSA put the review on hold. Neither agency responded.
It is possible the Biden administration was waiting for the conclusion of oral arguments in federal circuit courts in six 340B contract pharmacy lawsuits before deciding whether to issue the replacement ADR proposed rule for notice and comment. The last of three federal appeals courts hearing those cases held arguments on Tuesday.
This is a developing story. Check 340B Report later today for possible updates.